A kind of graphene antihypertensive patch and preparation method thereof
A graphene and blood pressure-lowering technology, which is applied in the direction of pharmaceutical formulations, other medical devices, cardiovascular system diseases, etc., can solve problems such as toxic side effects, impact on human health, and impact, and achieve a good blood pressure lowering effect and enhance the body's immunity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
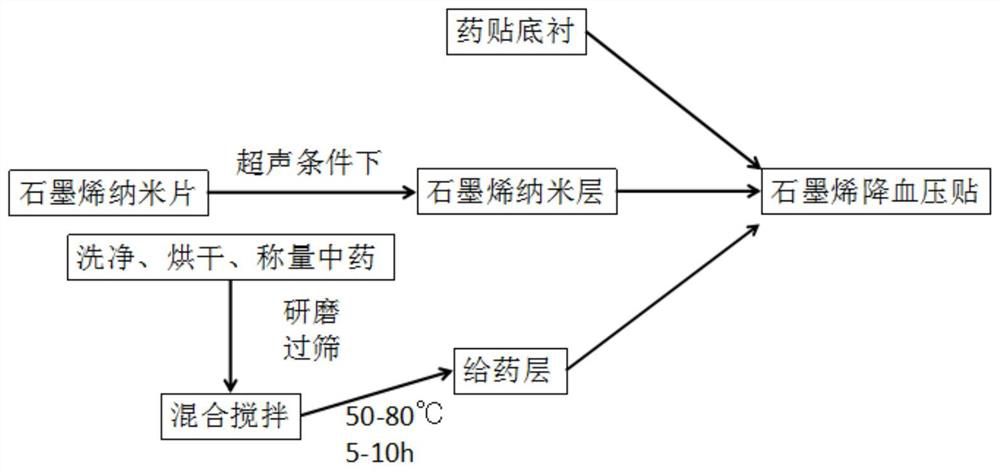
[0028] Embodiment 1: a kind of graphene antihypertensive patch, comprises medicine patch bottom liner and administration layer, is coated with the first layer of graphene nano-layer on the medicine patch bottom liner, is coated with drug administration layer on the first layer of graphene nano-layer layer, the drug delivery layer is coated with a second layer of graphene nano-layers, wherein the drug delivery layer includes the following components by weight of traditional Chinese medicine:
[0029] 30 parts of Cyclocarya paliurus; 25 parts of Mulberry; 18 parts of Chuanxiong; 15 parts of Pueraria lobata; 8 parts of wild chrysanthemum; 6 parts of dandelion; 7 parts of camellia oil; 5 parts of Moutan bark; 4 parts of Poria; ; 2 parts of mulberry leaves; 1 part of osmanthus leaves.
[0030] The first layer of graphene nanolayer and the second layer of graphene nanolayer include the following components by weight: 80 parts of graphene; 5 parts of 2-(3,4-epoxycyclohexane) ethyltri...
Embodiment 2
[0036] Embodiment 2: A kind of graphene antihypertensive patch comprises a drug patch backing and a drug delivery layer, the first layer of graphene nano-layer is coated on the drug patch backing, and the first layer of graphene nano-layer is coated with a drug delivery layer. layer, the drug delivery layer is coated with a second layer of graphene nano-layers, wherein the drug delivery layer includes the following components by weight of traditional Chinese medicine:
[0037]20 parts of Cyclocarya paliurus; 20 parts of Mulberry; 10 parts of Rhizoma Chuanxiong; 10 parts of Pueraria lobata; 5 parts of wild chrysanthemum; 5 parts of dandelion; 5 parts of camellia oil; 3 parts of Moutan bark; ; 1 part mulberry leaf; 1 part laurel leaf
[0038] The first layer of graphene nanolayer and the second layer of graphene nanolayer include the following components by weight: 80 parts of graphene; 5 parts of 2-(3,4-epoxycyclohexane) ethyltrimethoxysilane; 2 parts of triethoxy-1H,1H,2H,2H-...
Embodiment 3
[0044] Embodiment 3: A kind of graphene lowering blood pressure sticks, comprises medicine patch backing and administration layer, is coated with the first layer of graphene nano-layer on the medicine patch backing, is coated with drug-administering layer on the first layer of graphene nano-layer layer, the drug delivery layer is coated with a second layer of graphene nano-layers, wherein the drug delivery layer includes the following components by weight of traditional Chinese medicine:
[0045] 40 parts of Cyclocarya paliurus; 30 parts of Mulberry; 20 parts of Chuanxiong; 20 parts of Pueraria lobata; 10 parts of wild chrysanthemum; 8 parts of dandelion; 8 parts of camellia oil; 5 parts of Moutan bark; ; 2 parts of mulberry leaves; 2 parts of osmanthus leaves.
[0046] The first layer of graphene nanolayer and the second layer of graphene nanolayer include the following components by weight: 80 parts of graphene; 5 parts of 2-(3,4-epoxycyclohexane) ethyltrimethoxysilane; 2 p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fineness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com