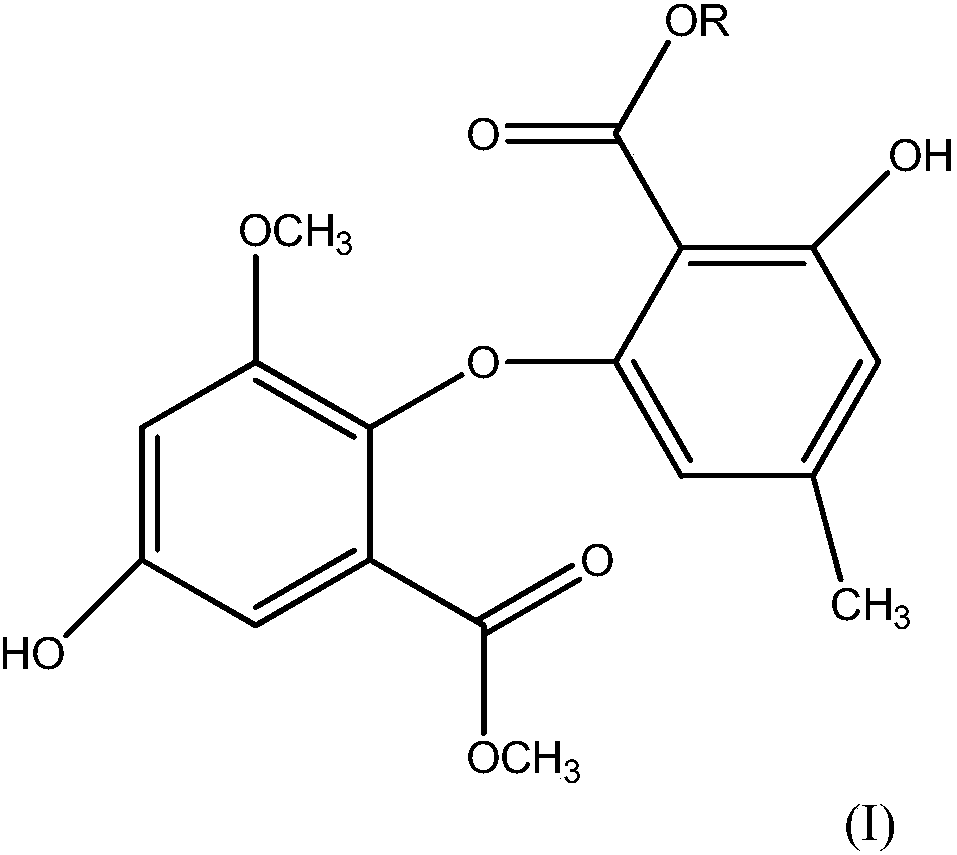
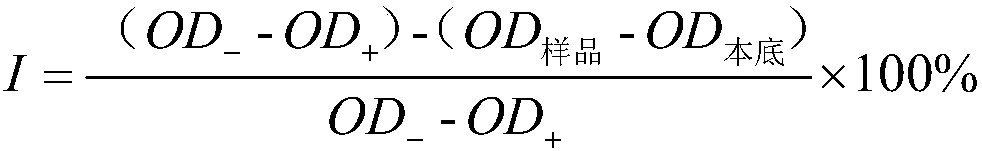Application of asterric acids compound in inhibition of activity of acetylcholin esterase
A technology of acetylcholinesterase and compounds, which is applied in the field of medicine and can solve problems not specifically related to the activity of acetylcholinesterase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
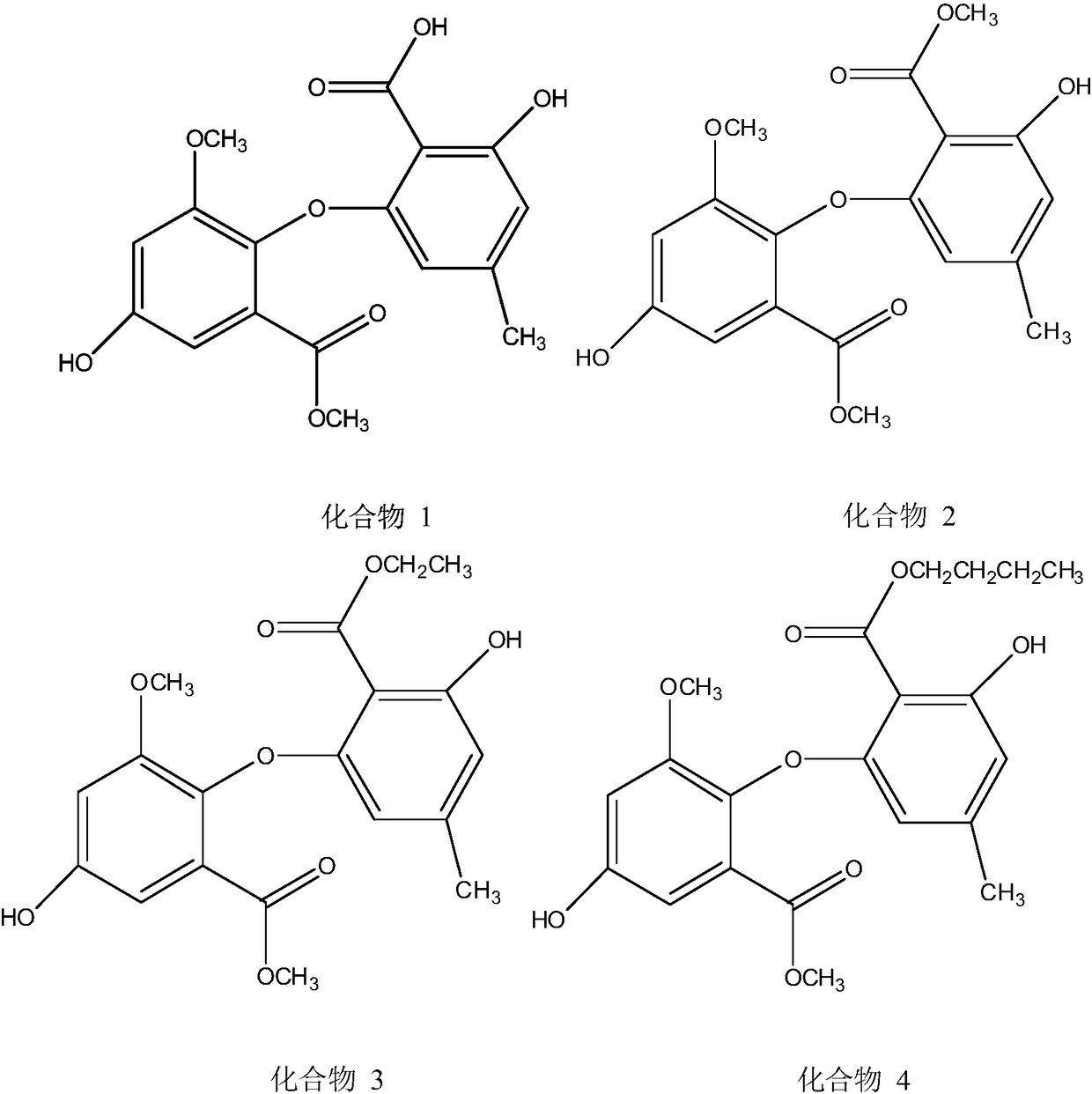
[0015] Embodiment 1: In vitro inhibitory activity test of acetylcholinesterase of compound 1
[0016] (1) Drugs and reagents
[0017] Acetylcholinesterase (AChE, Sigma Company), 50mmol / L, pH8.0 phosphate-buffered saline (PBS), 15mmol / L5,5-dithio-2-nitrobenzoic acid (dithiobis-nitrobenzoic acid, DTNB) Solution (Sigma Company) (prepared with 50mmol / L, pH 8.0PBS), 15mmol / L thioacetylcholine (Acetyl thiocholine, ATch, Sigma Company, prepared with 50mmol / L, pH 8.0PBS), 4% lauryl sulfate Sodium (SDS, Sigma Company), positive control Huperzine A (National Institute for the Control of Pharmaceutical and Biological Products), 0.75mol / L MgCl 2 Solution, methanol is analytically pure.
[0018] (2) Experimental method
[0019] Refer to the literature (Zhang Yi, Feng Yan, Li Xiaoming, et al. Research on the inhibition of acetylcholinesterase activity by seaweed components [J]. Ocean and Limnology, 2005,36(5):459-464.) to make appropriate improvements. The reference substance for the de...
Embodiment 2
[0026] Embodiment 2: In vitro inhibitory activity test of acetylcholinesterase of compound 2
[0027] (1) Drugs and reagents
[0028] Acetylcholinesterase (AChE, Sigma Company), phosphate-buffered saline (PBS) at 50mmol / L, pH8.0, 15mmol / L 5,5-dithio-2-nitrobenzoic acid (dithiobis-nitrobenzoic acid, DTNB ) solution (Sigma Company) (prepared with 50mmol / L, pH 8.0PBS), 15mmol / L thioacetylcholine (Acetyl thiocholine, ATch, Sigma Company, prepared with 50mmol / L, pH 8.0PBS), 4% dodecyl Sodium sulfate (SDS, Sigma company), positive control Huperzine A (National Institute for the Control of Pharmaceutical and Biological Products), 0.75mol / L MgCl 2 Solution, methanol is analytically pure.
[0029] (2) Experimental method
[0030] Refer to the literature (Zhang Yi, Feng Yan, Li Xiaoming, et al. Research on the inhibition of acetylcholinesterase activity by seaweed components [J]. Ocean and Limnology, 2005,36(5):459-464.) to make appropriate improvements. The reference substance for ...
Embodiment 3
[0037] Embodiment 3: In vitro inhibitory activity test of acetylcholinesterase of compound 3
[0038] (1) Drugs and reagents
[0039] Acetylcholinesterase (AChE, Sigma Company), phosphate-buffered saline (PBS) at 50mmol / L, pH8.0, 15mmol / L 5,5-dithio-2-nitrobenzoic acid (dithiobis-nitrobenzoic acid, DTNB ) solution (Sigma Company) (prepared with 50mmol / L, pH 8.0PBS), 15mmol / L thioacetylcholine (Acetyl thiocholine, ATch, Sigma Company, prepared with 50mmol / L, pH 8.0PBS), 4% dodecyl Sodium sulfate (SDS, Sigma company), positive control Huperzine A (National Institute for the Control of Pharmaceutical and Biological Products), 0.75mol / L MgCl 2 Solution, methanol is analytically pure.
[0040] (2) Experimental method
[0041] Refer to the literature (Zhang Yi, Feng Yan, Li Xiaoming, et al. Research on the inhibition of acetylcholinesterase activity by seaweed components [J]. Ocean and Limnology, 2005,36(5):459-464.) to make appropriate improvements. The reference substance for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com