A method for maximizing the production of chemical products using catalytic cracking gasoline
A technology for catalytic cracking of gasoline and chemical products, which is applied to hydrocarbon oil treatment products, petroleum industry, and hydrocarbon oil treatment. It can solve the problems of inability to convert into chemical products, achieve good industrial scale-up adaptability, increase aromatics content, and maintain long-term The effect of cycle stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1. Preparation of aromatization catalyst
[0067] 1) Preparation of catalyst precursor
[0068] At room temperature, the nanoscale HZSM-5 molecular sieve with a silicon-to-aluminum ratio of 25 and pseudo-boehmite were physically mixed in a ratio of 4:1 to obtain a catalyst precursor.
[0069] 2) Ion exchange modification
[0070] The catalyst precursor was ion-exchanged by the constant temperature water bath method, specifically, sodium hydroxide was dissolved in deionized water, mixed with the catalyst precursor and placed in a water bath at 90°C for 2 hours, so that the loading capacity of sodium was about 0.2 % by weight, followed by drying at about 120°C for about 8 hours and firing at about 540°C for about 4 hours.
[0071] 3) The first modification treatment
[0072]Using the equal-volume impregnation method, the first modification treatment is carried out on the catalyst precursor treated by ion exchange, specifically, dissolving ammonium dihydrogen phosphate ...
Embodiment 2
[0089] 1. Preparation of aromatization catalyst
[0090] 1) Preparation of catalyst precursor
[0091] At room temperature, the nanoscale HZSM-5 molecular sieve with a silicon-to-aluminum ratio of 25 and pseudo-boehmite were physically mixed at a ratio of 9:1 to obtain a catalyst precursor.
[0092] 2) Ion exchange modification
[0093] The catalyst precursor was ion-exchanged by using a constant temperature water bath method, specifically dissolving sodium hydroxide in deionized water, mixing it with the catalyst precursor, and stirring it in a water bath at 90°C for 2 hours, so that the loading capacity of sodium was about 0.5 % by weight, followed by drying at about 120°C for about 8 hours and firing at about 540°C for about 4 hours.
[0094] 3) The first modification treatment
[0095] Using an equal-volume impregnation method, carry out the first modification treatment on the ion-exchange-treated catalyst precursor, specifically dissolving lanthanum nitrate in deionize...
Embodiment 3
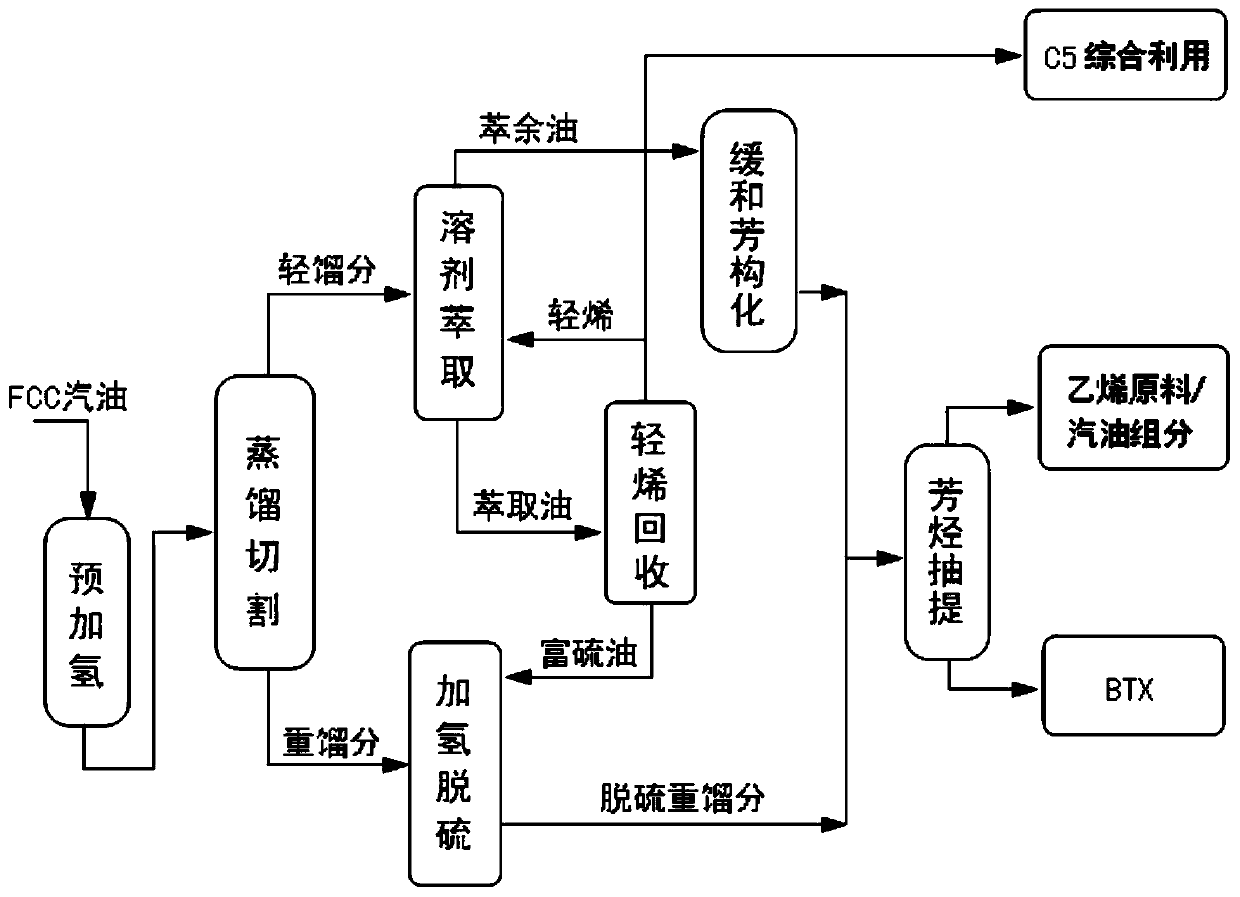
[0111] Such as figure 1 As shown, the method of utilizing catalytic cracking gasoline to maximize the production of chemical products in this embodiment includes the following steps:
[0112] 1. Prehydrogenation
[0113] The composition of the catalytic cracking gasoline raw material in this embodiment is shown in Table 5.
[0114] Table 5 Composition of FCC gasoline feedstock
[0115]
[0116] In the presence of a pre-hydrogenation catalyst, the above catalytic cracked gasoline is pre-hydrogenated to obtain a pre-hydrogenation catalytic cracked gasoline; wherein the pre-hydrogenation catalyst is a nickel-molybdenum bimetallic catalyst, which consists of (mass content%): Al 2 o 3 90.5%, Ni6%, Mo 3.5%; the pre-hydrogenation process conditions are: control the reaction temperature at 130°C, the hydrogen-oil ratio is 5, and the volume space velocity is 3h -1 .
[0117] After the above-mentioned pre-hydrogenation, the light sulfur compounds in the catalytic cracking gasol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com