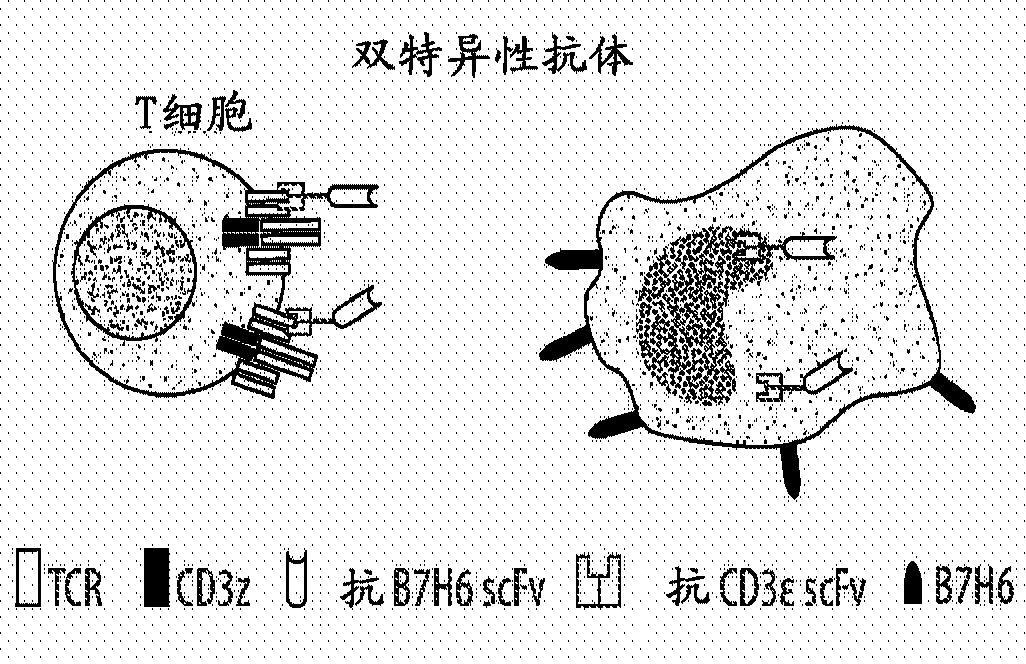
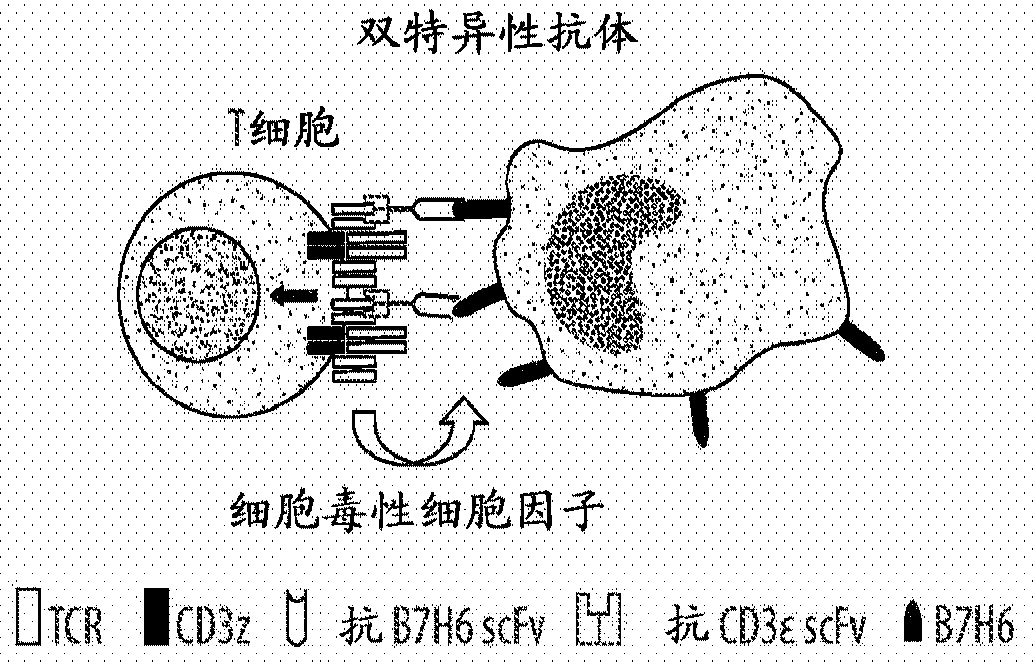
Tumor immunotherapy
An engineering, circuit technology with applications in the fields of synthetic biology and immunology to address the problems of incurable metastatic disease, morbidity and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Two human promoters were used as promoter inputs for the engineered genetic circuits used in this example. These human promoters SSX1 (input 1) and H2A1 (input 2) are overexpressed in many human cancers (input 1 encodes mKate2 output containing intervening mirFF4) ( Figure 4A ). Measurement of different circuit configurations relative to (a) the number of perfectly matched mirFF4 binding sites (FF4-BS) encoded in input 1 (downstream of mK2) and (b) two different configurations of the 'sponge' construct in input 2 mKate2 output level. Figure 4B , x-axis annotation: M# represents the number of mirFF4 binding sites (FF4-BS) encoded downstream of mKate2 / mirFF4 that input 1 has. For example, M3 represents input 1 with 3 perfectly matching mirFF4 binding sites (FF4-BS) ( Figure 4A ). S0, S1 and S2 represent three different sponge / input 2 configurations. S0 is a negative control transcript that does not have a mirFF4 binding site. S1 is a decoy transcript (Decoy trans...
Embodiment 2
[0173] The engineered genetic circuit (G5) described in this example is based on the mKate2-encoding circuit (AND gate) described in Example 1, with the difference that the product of the AND gate is not mKate2 but a synthetic transcription factor (in Figure 6A are noted as "TF"). Although TF can be any transcriptional activator, such as rtTA3, TALE-TF, and ZF-TF, in this example, TF is the fusion protein GAL4BD-VP16AD (yeast GAL4 DNA-binding domain fused to viral VP16 transcriptional activation domain ). Alternatively, this could also be a transcriptional repressor, eg GAL4BD-KRAB. Because the export is a transcription factor rather than a reporter / effector protein, it can regulate the expression of multiple exports encoded downstream of the TF target promoter. In this example, the target promoter (annotated as P3) was a synthetic G5 promoter consisting of a minimal viral or human promoter with 5 upstream GAL4 DNA binding sites. The I / O profile of the synthetic promoter c...
Embodiment 3
[0175] Example 3. BiTEs and STEs Trigger Robust Tumor Killing
[0176] HEK-293T cells
[0177] Anti-HER2 bispecific T cell engager (BiTE) and surface T cell engager (STE) trigger T cells to mediate robust tumor killing and IFN-γ secretion ( Figure 8 ). HEK-293T (minimally expressing HER2) cells were transfected with various DNA constructs as indicated. 48 hours after transfection, various HEK-293T cells were harvested and co-cultured with human T cells for 5 hours or 24 hours. The 5-hour cytotoxicity of T cells was measured by lactate dehydrogenase (LDH) release assay (Korzeniewski C and Callewaert DM, Journal of Immunological Methods, 1983, 64(3):313-320, incorporated herein by reference ), and 24-h IFN-γ secretion by T cells was measured by IFN-γ ELISA. The data indicate that T cells mediate robust tumor killing and IFN-γ secretion against BiTE-secreting tumor cells (Groups 1-2). Tumor killing and IFN-γ secretion correlated with HER2 expression levels on tumor cells (G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com