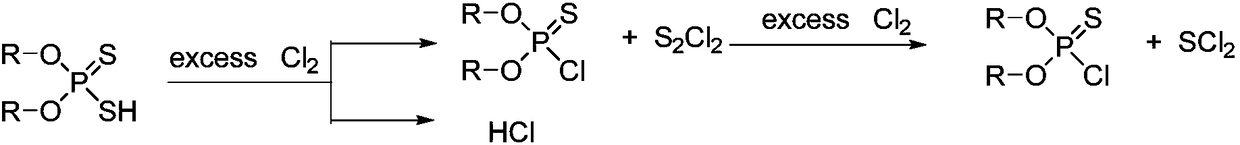
Synthesis method of O, O-dialkyl thiphosphoryl chloride
The technology of an alkyl thiophosphoryl and a synthesis method is applied in the field of preparation of pesticide intermediates, can solve the problems of three wastes and the like, and achieves the effects of improving safety and environmental protection level, low comprehensive cost and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]Add 329.3g (2mol) methyl sulfide (96%) and 0.6g Iron powder catalyst, feed chlorine gas at a controlled temperature of 40°C, and absorb the hydrogen chloride produced with water. The system gradually became cloudy, producing a yellow solid. When about 150 g of chlorine gas is introduced, the system is turned to a pressure of -0.04 MPa and the reaction temperature is maintained at 40°C. With the introduction of chlorine gas, the yellow solid gradually disappears, and brown-red sulfur dichloride is collected by rectification and condensation. Chlorine gas was fed for 5 hours (total feed time), and no obvious liquid was collected when 319 g (4.4 mol, total feed amount) was fed, and the reaction was stopped. A total of 210 g of sulfur dichloride was collected. The chlorine gas flow was stopped, and high vacuum rectification at -0.099 MPa obtained 303.8 g of O, O-dimethylphosphorylthiochloride (purity 99%), yield 94.0%.
Embodiment 2
[0043] Add 391.6g (2mol) ethyl sulfide (96%) and 1.0g Iron powder catalyst, feed chlorine gas at a controlled temperature of 45°C, and absorb the hydrogen chloride produced with water. The system gradually became cloudy, producing a yellow solid. When about 160g of chlorine gas is fed in, the system is turned to a pressure of -0.04MPa while maintaining a reaction temperature of 45°C. The yellow solid gradually disappears as the chlorine gas is fed in, and brown-red sulfur dichloride is collected by rectification and condensation. Chlorine gas was passed in for 4.5 hours, and no obvious liquid was collected when 306 g (4.3 mol) was passed in, so the reaction was stopped. A total of 205 g of sulfur dichloride was collected. Chlorine flow was stopped, and high vacuum rectification at -0.099MPa gave 355.7g of O,O-diethylphosphorylthiochloride (purity 99%), yield 93.4%.
Embodiment 3
[0045] Add 329.3g (2mol) methyl sulfide (96%) and 0.4g Iron powder catalyst, feed chlorine gas at 30°C, and absorb the generated hydrogen chloride with water. The system gradually became cloudy, producing a yellow solid. When about 170g of chlorine gas is introduced, the system is turned to a pressure of -0.05MPa while maintaining a reaction temperature of 30°C. With the introduction of chlorine gas, the yellow solid gradually disappears, and brown-red sulfur dichloride is collected by rectification and condensation. Chlorine gas was passed through for 5 hours, and when 355 g (5.0 mol) was passed through, no obvious liquid was collected, and the reaction was stopped. A total of 207 g of sulfur dichloride was collected. Chlorine flow was stopped, and high vacuum rectification at -0.099MPa gave 302.6g of O,O-dimethylphosphorylthiochloride (purity: 99%), with a yield of 93.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com