Method for preparing 2, 3-dimethyl-2H-indazole-6-benzylamine hydrochloride
A dimethyl, dimethyl sulfoxide technology, applied in chemical instruments and methods, organic chemistry, chemical/physical/physical chemical processes, etc., can solve the problems of dangerous reaction process, environmental pollution, long time consumption, etc. Simple and convenient handling, low toxicity and pollution, good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
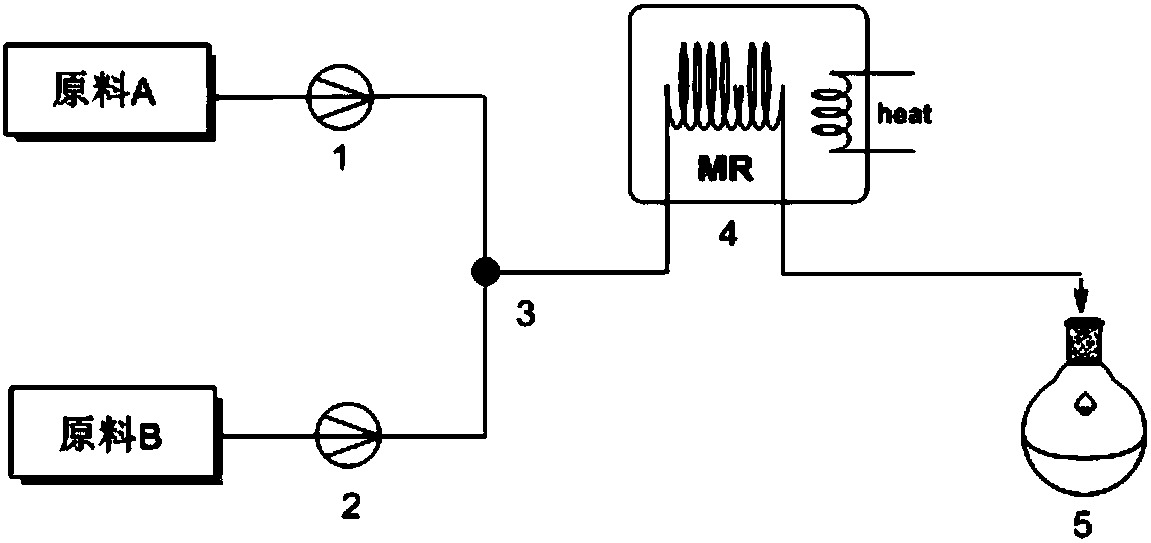
[0030] Such as figure 1 , the first microchannel reaction device includes a pump A 1 , a pump B 2 , a first mixing valve 3 , a first microreactor 4 , and a first receiving device 5 . The pump A and the pump B are connected in parallel through the connecting pipe and the first mixing valve, and the first mixing valve, the first microreactor and the first receiving device are connected in series through the connecting pipe.
[0031] Such as figure 2, the second continuous microchannel reaction device includes pump C 6, pump D 7, the second mixing valve 8, the second microreactor 9, pump E 10, the third mixing valve 11, the third microreactor 12, the second Receiver 13. Pump C and pump D are connected in parallel through the connecting pipe and the second mixing valve, the second mixing valve and the second microreactor are connected in series through the connecting pipe, and the second microreactor and pump E are connected in parallel through the connecting pipe It is connec...
Embodiment 2
[0041] The operation is the same as in Example 1, the only difference is:
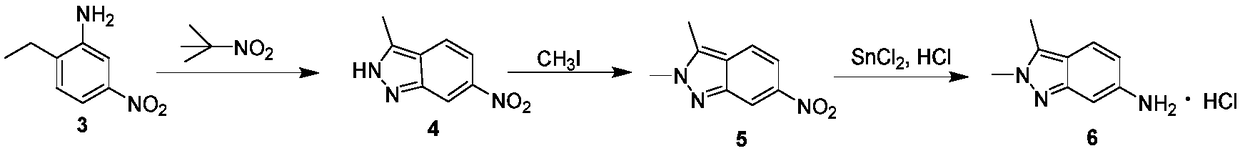
[0042] In step (1), the glacial acetic acid solution concentration of tert-butyl nitrite is 0.5mol / L, and the glacial acetic acid solution concentration of 5-nitro-2-ethylaniline is 0.067mol / L; -The mol ratio of nitro-2-ethylaniline is 1:1.5; The flow velocity of pump A is 0.11mL / min, and the flow velocity of described pump B is 0.85mL / min; The volume of the first microreactor is 5mL, The residence time of the reaction was 52 min.
[0043] In step (2), the dimethyl sulfoxide solution concentration of 3-methyl-6-nitro-1H-indazole is 0.51mol / L, and the dimethyl sulfoxide solution concentration of methyl iodide is 1.47mol / L, The concentration of the dimethyl sulfoxide solution of sodium ethoxide is 0.31mol / L. The molar ratio of 3-methyl-6-nitro-1H-indazole to methyl iodide is 1:2.7; the molar ratio of 3-methyl-6-nitro-1H-indazole to sodium ethoxide is 1:3. The flow rate of pump C is 0.21-0.806mL / min, t...
Embodiment 3
[0046] The operation is the same as in Example 1, the only difference is:
[0047] In step (1), the glacial acetic acid solution concentration of tert-butyl nitrite is 2.5mol / L, and the glacial acetic acid solution concentration of 5-nitro-2-ethylaniline is 0.333mol / L; -The mol ratio of nitro-2-ethylaniline is 1:4; The flow velocity of pump A is 0.22mL / min, and the flow velocity of described pump B is 1.65mL / min; The first microreactor volume is 50mL, The residence time of the reaction was 2.7 min.
[0048] In step (2), the dimethyl sulfoxide solution concentration of 3-methyl-6-nitro-1H-indazole is 2.73mol / L, and the dimethyl sulfoxide solution concentration of described methyl iodide is 7.86mol / L L, the concentration of the dimethyl sulfoxide solution of sodium ethoxide is 1.69mol / L. The molar ratio of 3-methyl-6-nitro-1H-indazole to methyl iodide is 1:10; the molar ratio of 3-methyl-6-nitro-1H-indazole to sodium ethoxide is 1:8.5. The flow rate of pump C is 0.806mL / min, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com