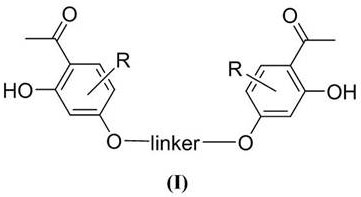
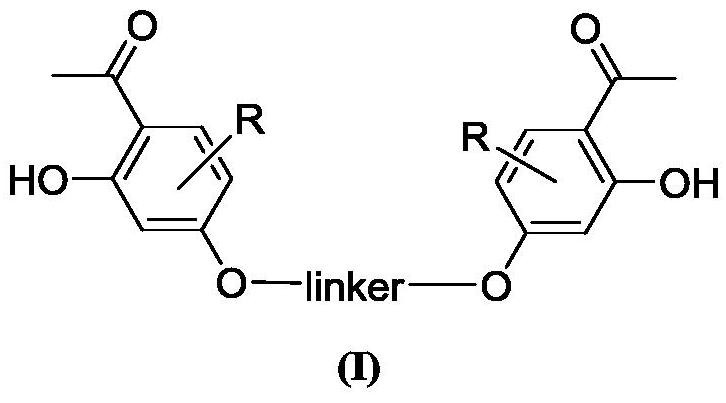
A kind of bis-o-hydroxyacetophenone gemini compound and its preparation method and application
A technology of o-hydroxyacetophenone and hydroxyacetophenone is applied in the field of di-o-hydroxyacetophenone twin drug compounds and their preparation, and can solve the problems of many toxic and side effects, poor long-term efficacy of AD patients, complex etiology of AD and the like , to achieve the effect of strong antioxidant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
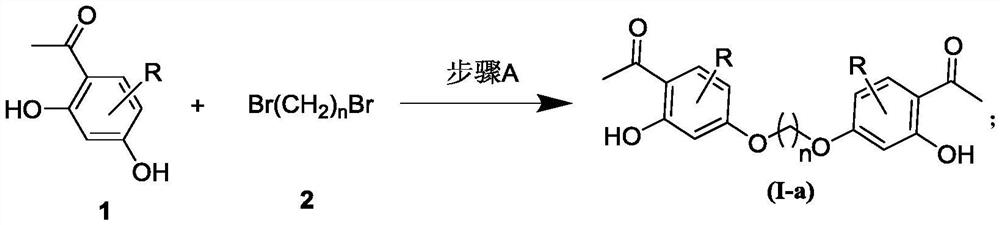
[0032] General method for the preparation of embodiment 1 bis-o-hydroxyacetophenone gemini drug compound Ia
[0033] Add 2.0mmol of 2-hydroxyacetophenone (1), 10mmol of the corresponding dibromocompound (2), 3mmol of anhydrous potassium carbonate and 50ml of acetonitrile in the reaction flask, after stirring evenly, heat up and reflux and stir for 2.0 to 72 hours ( The reaction process is tracked by TLC); after the reaction, evaporate the solvent to dryness under reduced pressure, add 80ml of deionized water, extract three times with 200mL of dichloromethane, wash with saturated sodium chloride after the organic layer is combined, dry and filter through anhydrous sodium sulfate , two 2-hydroxyacetophenone gemini compounds (Ia) are evaporated to dryness under reduced pressure, and the residue is purified by column chromatography (petroleum ether: acetone=100:1v / v) to obtain the corresponding two 2-hydroxyacetophenone Ketogemide compound Ia, the yield is 15%-65%, and its chemica...
Embodiment 2
[0054] General method for the preparation of embodiment 2 two o-hydroxyacetophenone gemini compounds (Ib)
[0055]Add 2.0mmol of 2-hydroxyacetophenone (1), 10mmol of the corresponding dibromoalkylamine compound (3), 5mmol of anhydrous potassium carbonate and 20ml of acetonitrile in the reaction flask, stir evenly, and then heat up and reflux to stir for 2.0 to 72 hours (the reaction process was tracked by TLC); after the reaction, the solvent was evaporated under reduced pressure, 80ml of deionized water was added, extracted three times with 200mL of dichloromethane, the organic layers were combined and washed with saturated sodium chloride, washed with anhydrous sodium sulfate Drying and filtering, two 2-hydroxyacetophenone gemini compounds (Ib) are evaporated to dryness under reduced pressure, and the residue is purified by column chromatography (petroleum ether: acetone=100:1v / v) to obtain the corresponding two 2-hydroxyl Acetophenone gemini compound (Ib), yield 10%-55%, it...
Embodiment 3
[0061] General method for the preparation of embodiment 3 two o-hydroxyacetophenone gemini compounds (Ic)
[0062] Add 2.0mmol of 2-hydroxyacetophenone (1), 10mmol of the corresponding ethyl bromoalkylate (4), 5mmol of anhydrous potassium carbonate and 20ml of acetonitrile in the reaction flask, stir evenly, and then raise the temperature and reflux to stir for 2.0-72 hours (the reaction process was tracked by TLC); after the reaction, the solvent was evaporated under reduced pressure, 80ml of deionized water was added, extracted three times with 200mL of dichloromethane, the organic layers were combined and washed with saturated sodium chloride, washed with anhydrous sodium sulfate Dry and filter, evaporate the solvent from ethyl ferulamide-O-alkanoate under reduced pressure, and purify the residue by column chromatography (petroleum ether: acetone=100:1v / v) to obtain the corresponding ferulamide-O-alkane Acetate ethyl ester, then add 20mL methanol and 10mmol30% LiOH solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com