Method for preparing trans-1,2-dichloroethylene
A dichloroethylene and cis technology, applied in the field of preparation of trans 1,2-dichloroethylene, can solve problems such as large output of trichloroethylene, and achieve the effects of simple process, easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] A preparation method of trans-1,2-dichloroethylene of the present invention comprises the following steps: cis-1,2-dichloroethylene is isomerized under the action of a transposition catalyst to obtain trans-1,2-dichloroethylene , the reaction temperature is 150-250°C, the residence time is 0.5-5s, the reaction pressure is normal pressure, and the product formed by the reaction is purified by rectification to obtain trans-1,2-dichloroethylene, and the transposition catalyst is Al 2 o 3 The main catalyst is a metal additive added to the main catalyst, wherein the metal additive accounts for 0.5% to 5%. Preferably, the metal additive is one of Ti, Cr, Fe, Ni and Zn. Preferably, the reaction The temperature is 180-230°C. The reaction temperature has a great influence on the performance of the conversion catalyst. If the reaction temperature is too high, the conversion rate of cis-1,2-dichloroethylene will increase, but the conversion rate of trans-1,2-dichloroethylene will ...
Embodiment 1
[0017] A certain amount of Zn / Al 2 o 3 The transposition catalyst is loaded into the tubular reactor, and the auxiliary agent accounts for 1.5%. The cis-1,2-dichloroethylene is vaporized and injected into the tubular reactor for reaction. The reaction temperature is 220°C, and the residence time is In 2.5s, the reaction pressure was at normal pressure, and the crude product formed was collected by cooling, rectified and purified to obtain pure trans-1,2-dichloroethylene.
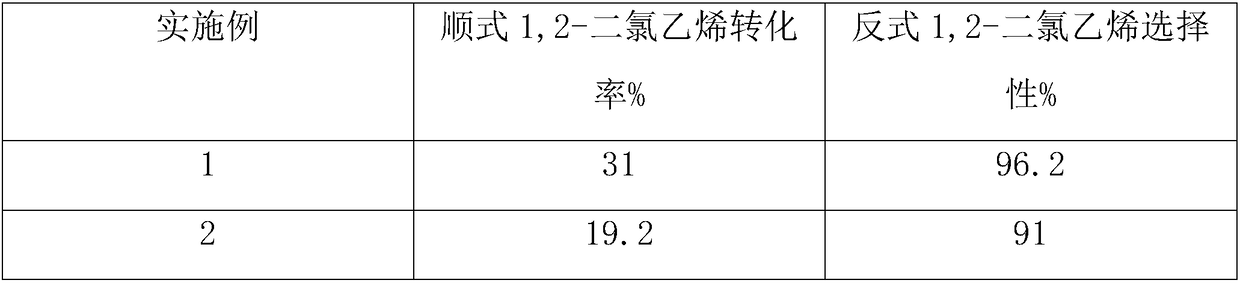
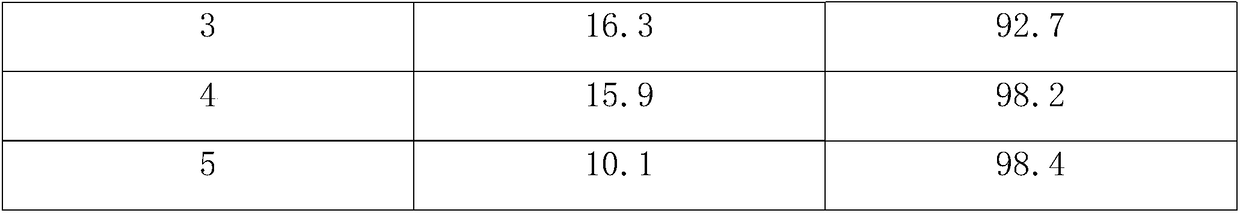
[0018] During the reaction, the cracked gas generated in the tubular reactor was analyzed, and the analysis results are shown in Table 1.
Embodiment 2
[0020] A certain amount of Ti / Al 2 o 3 The transposition catalyst is loaded into the tubular reactor, and the auxiliary agent accounts for 5%. The cis-1,2-dichloroethylene is vaporized and injected into the tubular reactor for reaction. The reaction temperature is 250°C, and the residence time is In 2.5s, the reaction pressure was at normal pressure, and the crude product formed was collected by cooling, rectified and purified to obtain pure trans-1,2-dichloroethylene.
[0021] During the reaction, the cracked gas generated in the tubular reactor was analyzed, and the analysis results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com