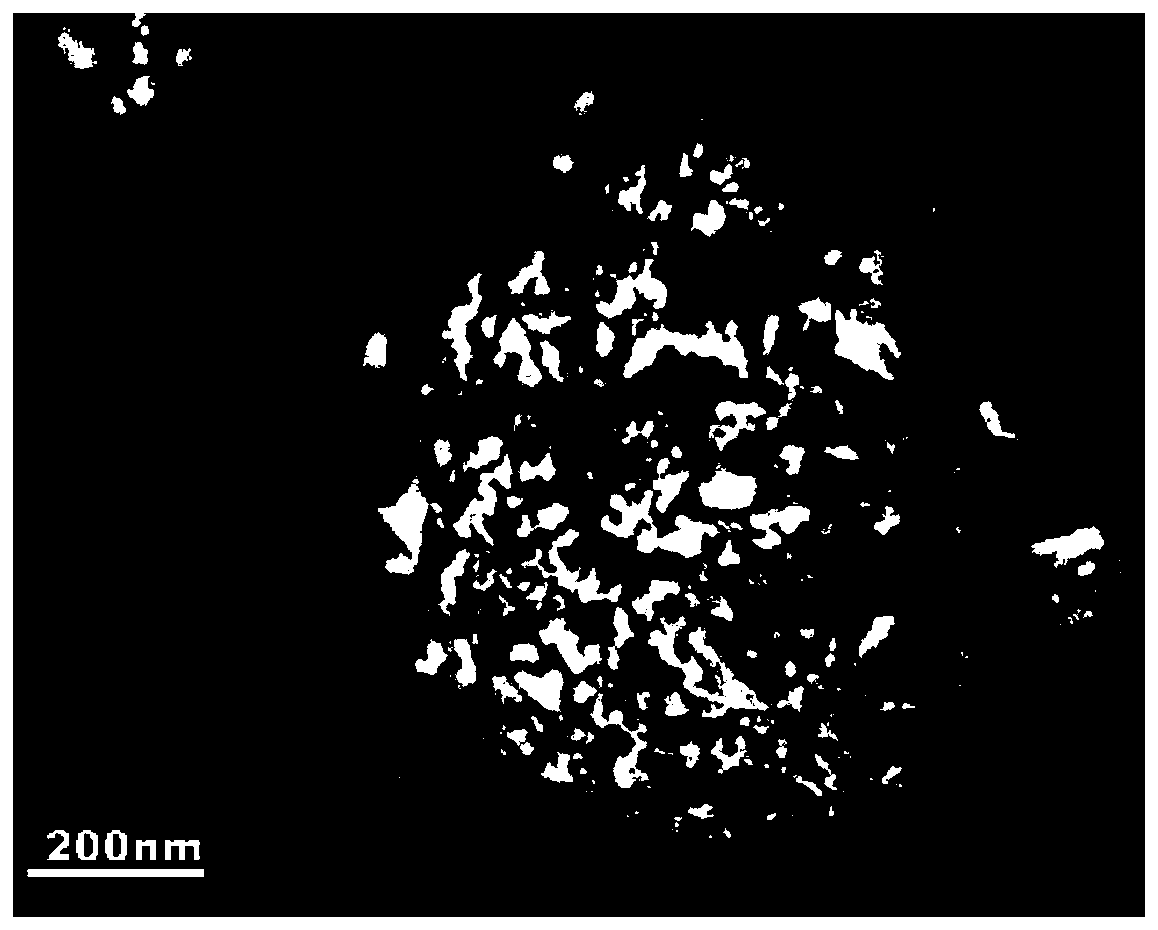
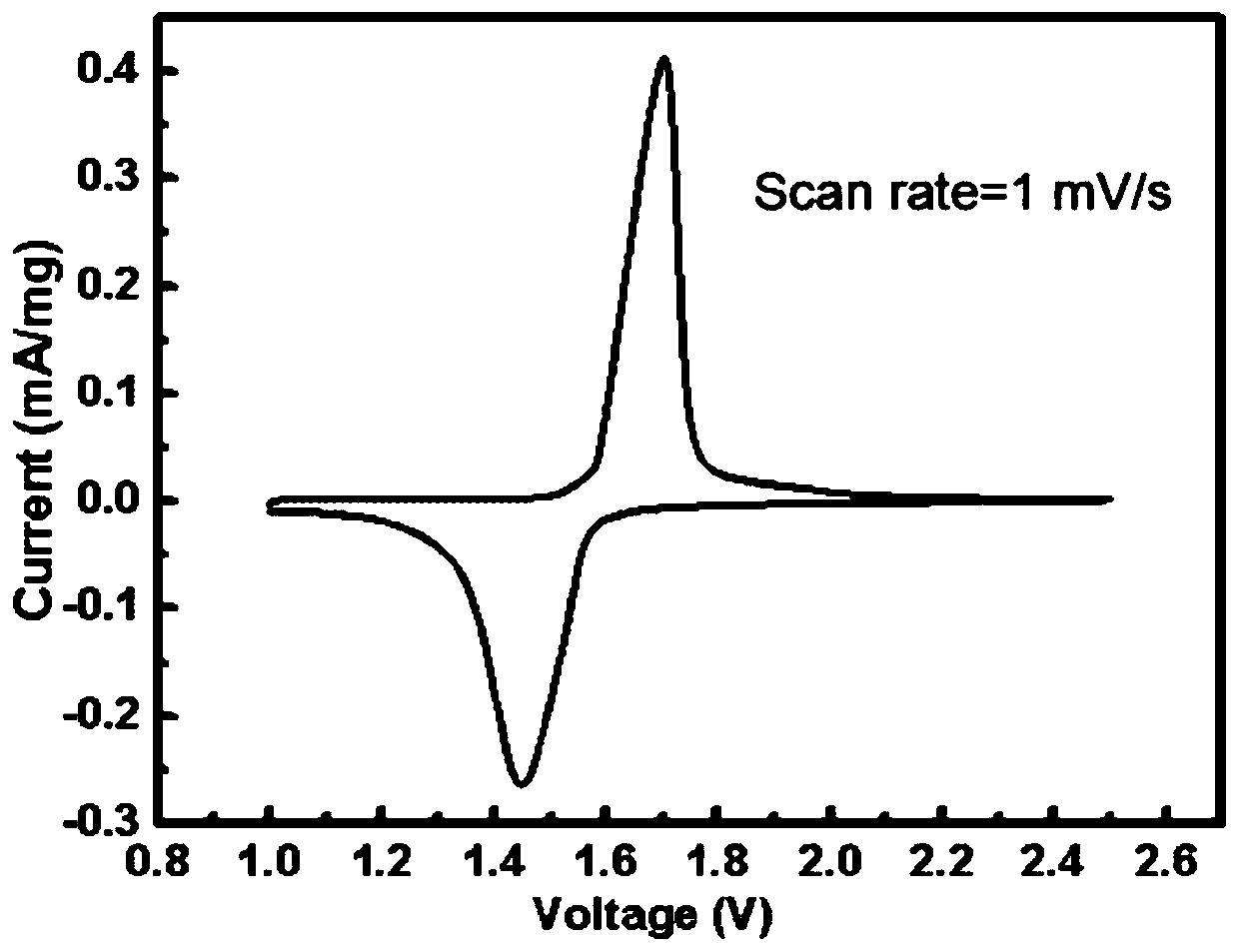
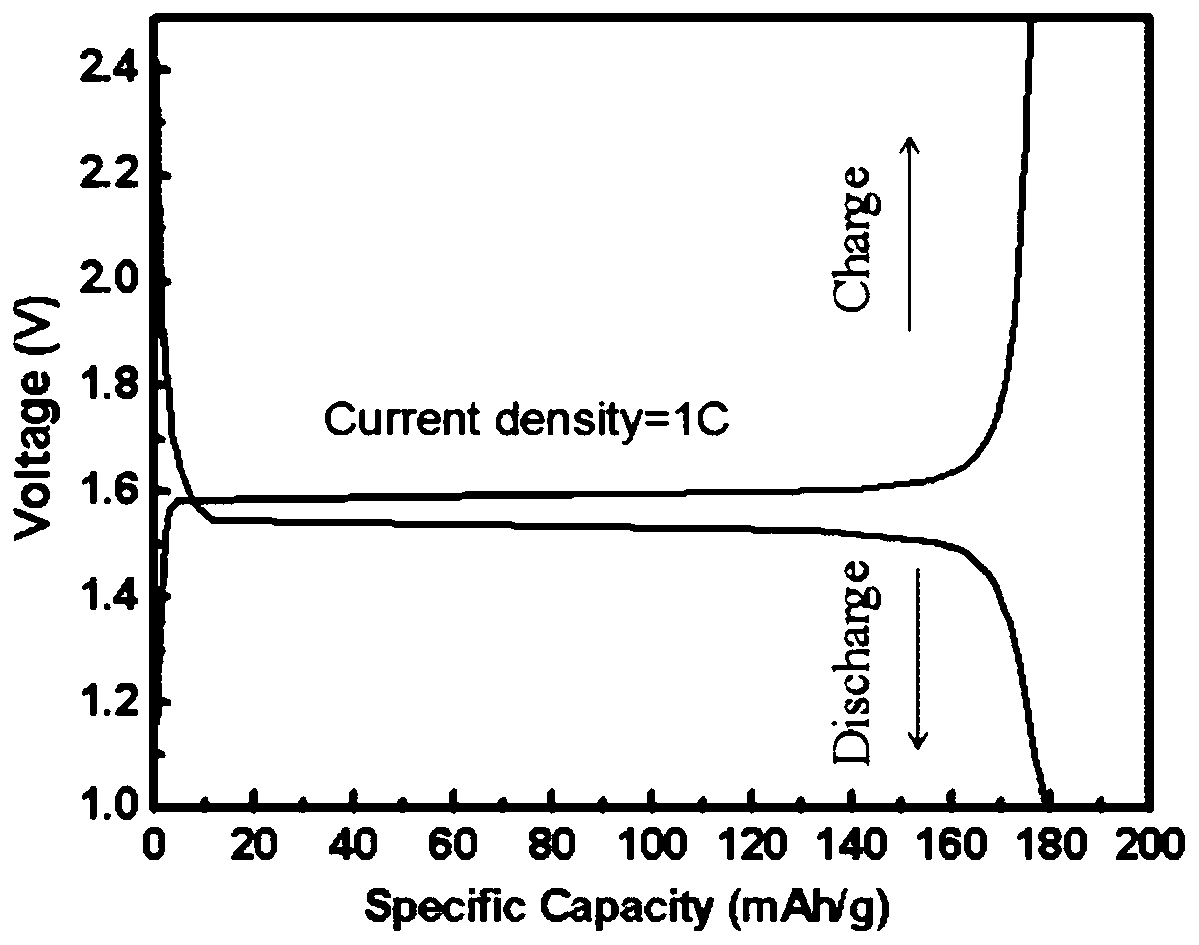
A preparation method of graphene-coated nitrogen-doped lithium titanate micro-nanospheres
A graphene-coated, heterolithic lithium titanate technology, which is applied in the manufacture of hybrid/electric double layer capacitors, hybrid capacitor electrodes, etc., can solve the problems of limited application, low specific capacity, poor electrical conductivity, etc., to improve electrochemical performance, Conductive network integrity, the effect of inhibiting secondary growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Disperse 4.2mL of tetrabutyl titanate in 30mL of ethanol, and gradually add it into a mixed system containing 30mL of ethanol and 2mL of deionized water at a rate of 0.2mL / s for hydrolysis for 1 hour to obtain Sol A.
[0038] (2) Dissolve 0.2g of dopamine hydrochloride in 100mL with a pH of 8.5 and a concentration of 1×10 -2 mol / L tris hydrochloric acid buffer solution, stirring and dissolving for 30s, to obtain solution B.
[0039] (3) 0.2 g of graphene oxide was ultrasonically dispersed in 200 mL of deionized water, and ultrasonically dispersed for 4 hours to obtain 1 g / L graphene oxide dispersion C.
[0040] (4) Add solution B to sol A at a rate of 100 mL / s, stir and react at 25° C. for 12 h to obtain sol D.
[0041] (5) Add dispersion C to sol D at a rate of 0.2mL / s. After stirring for 4 hours, add 0.4406g LiOH·H 2 O in 30mL lithium hydroxide solution, mix well and freeze-dry. Finally, the dried powder was calcined in a nitrogen atmosphere at 800 °C for 12 h,...
Embodiment 2
[0043] (1) Disperse 4.2mL of tetrabutyl titanate in 30mL of ethanol, and gradually add it into a mixed system containing 30mL of ethanol and 2mL of deionized water at a rate of 0.5mL / s for hydrolysis for 2 hours to obtain Sol A.
[0044] (2) Dissolve 0.2g of dopamine hydrochloride in 100mL with a pH of 6.5 and a concentration of 1×10 -2 mol / L Tris hydrochloric acid buffer solution, stirring and dissolving for 2 minutes to obtain solution B.
[0045] (3) 0.4 g of graphene oxide was ultrasonically dispersed in 200 mL of deionized water, and ultrasonically dispersed for 1 h to obtain 2 g / L graphene oxide dispersion C.
[0046] (4) Add solution B to sol A at a rate of 10 mL / s, stir and react at 25° C. for 24 hours to obtain sol D.
[0047] (5) Add dispersion C to sol D at a rate of 0.2mL / s. After stirring for 4 hours, add 0.4364g LiOH·H 2 O in 30mL lithium hydroxide solution, mix well and freeze-dry. Finally, the dried powder was calcined in a nitrogen atmosphere at 700° C. for...
Embodiment 3
[0049] (1) Disperse 4.2mL of tetrabutyl titanate in 30mL of ethanol, and gradually add it into a mixed system containing 30mL of ethanol and 4mL of deionized water at a rate of 0.2mL / s for hydrolysis for 2 hours to obtain Sol A.
[0050] (2) Dissolve 0.2g of dobutamine hydrochloride in 100mL with a pH of 6.5 and a concentration of 1×10 -2 mol / L tris hydrochloric acid buffer solution, stirring and dissolving for 30s, to obtain solution B.
[0051] (3) 0.2 g of graphene oxide was ultrasonically dispersed in 200 mL of deionized water, and ultrasonically dispersed for 1 hour to obtain 1 g / L graphene oxide dispersion C.
[0052] (4) Add solution B to sol A at a rate of 10 mL / s, stir and react at 25° C. for 12 h to obtain sol D.
[0053] (5) Add dispersion C to sol D at a rate of 1mL / s. After stirring for 4 hours, add 0.4406g LiOH·H 2 O in 30mL lithium hydroxide solution, mix well and freeze-dry. Finally, the dried powder was calcined in a nitrogen atmosphere at 800° C. for 20 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com