A kind of recombinant Artemisia annua Type 3 allergen protein and its application
An allergen-like, Artemisia annua technology, applied in the field of recombinant Artemisia annua class 3 allergen proteins and their coding genes and expression purification, can solve the problems of cumbersome process, pollution, complex composition, etc., and achieve simple preparation and purification process, Reduce allergic reaction, good immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 rArt a3 codon optimization
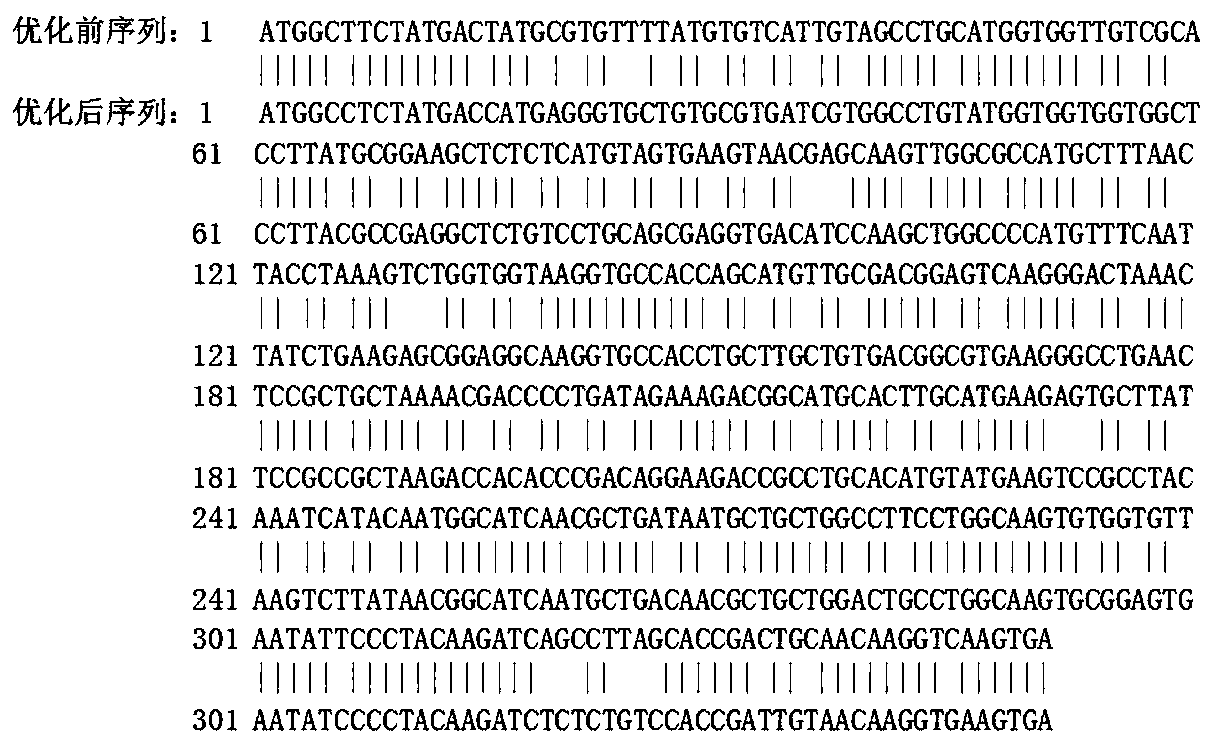
[0046] According to the mRNA sequence of Artemisia vulgaris clone1Art v3allergenprecursor (Art v3) published by NCBI (GenBank accession number: EU564846.1), the inventor obtained the cDNA sequence of Artemisia annua with high homology to Artv3 sequence but unknown gene function from NCBI EST ( NCBI accession number: GW332468.1), the sequence is shown in SEQ ID No: 2, the rArt a3 gene of the present invention is obtained after codon optimization of the gene, the nucleotide sequence is shown in SEQ ID No: 1, the amino acid sequence As shown in SEQ ID No:3. The following is a comparison of the parameters before and after rArt a3 codon optimization:
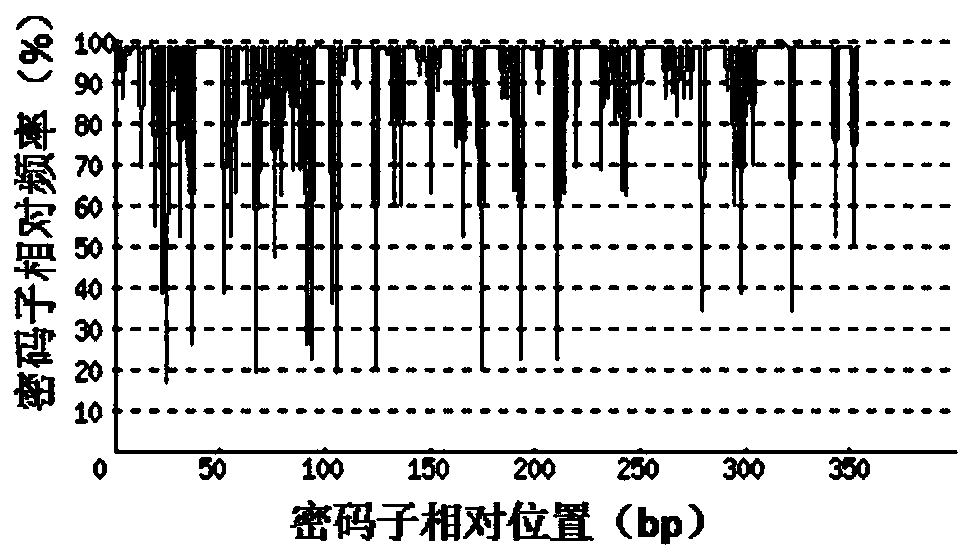
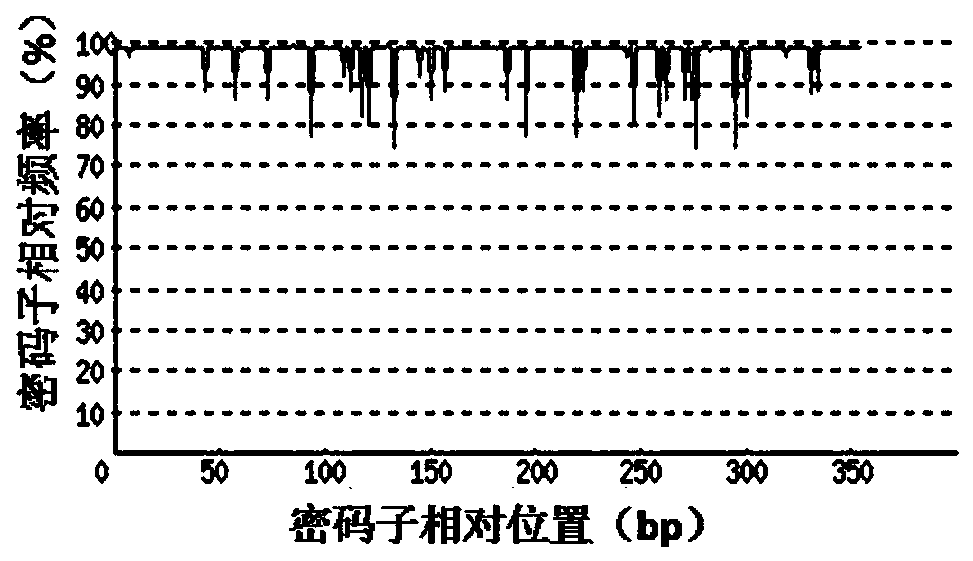
[0047] 1. Codon Adaptation Index (CAI)
[0048] Depend on Figure 2-a It can be seen that before codon optimization, the codon adaptation index (CAI) of rArt a3 original gene in mammalian cell CHO expression system was 0.72. Depend on Figure 2-b It can be seen that through codon o...
Embodiment 2
[0053] Embodiment 2: Containing the expression plasmid construction of rArt a3 gene
[0054] The codon-optimized rArt a3 gene was introduced into the AvrII restriction site sequence at the 5' end, and the BstZ17I restriction site sequence was introduced at the 3' end, and the whole gene synthesis was carried out, and the synthetic gene fragment was constructed into the pUC57 plasmid ( Provided by Nanjing GenScript Co., Ltd.), a long-term storage plasmid was obtained, which was designated as pUC57-rArt a3 plasmid.
[0055] The pUC57-rArt a3 plasmid was used as a template for PCR amplification, and the primer sequences used were as follows:
[0056] Upstream primer (SEQ ID No: 4):
[0057] M13F: TGT AAA ACG ACG GCC AGT
[0058] Downstream primer (SEQ ID No: 5):
[0059] M13R: CAG GAA ACA GCT ATG AC
[0060] The total reaction volume was 50 μL, in which 2.5 μL was added to each primer with a concentration of 10 μmol / L, and 1 μL was added to dNTP with a concentration of 10 m...
Embodiment 3
[0063] Example 3: Preparation of Mammalian Cell Line Containing Stable Expression of rArt a3 Gene
[0064] Puromycin is an aminoglycoside antibiotic that blocks protein synthesis in mammalian cells by interfering with ribosome function. The pac gene from Streptomyces has the effect of detoxifying Puromycin. The pCHO1.0 vector contains the pac gene, so Puromycin can be used as a screening antibiotic for pCHO1.0 as the expression vector. MTX is a folic acid antagonist, which can inhibit the activity of DHFR after conversion in cells, inhibit nucleic acid synthesis, and cause cytotoxicity. pCHO1.0 contains the DHFR gene, and MTX can be used as a screening reagent. The transfected cells contain Puromycin and MTX resistance genes, and the concentration of the screening reagent is continuously increased to increase the copy number of the target gene in the positive cells to increase the expression level.
[0065] The correctly sequenced pCHO-rArt a3 plasmid was linearized with N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com