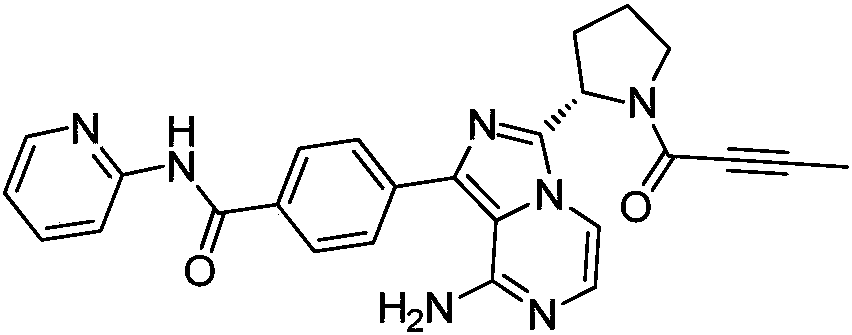
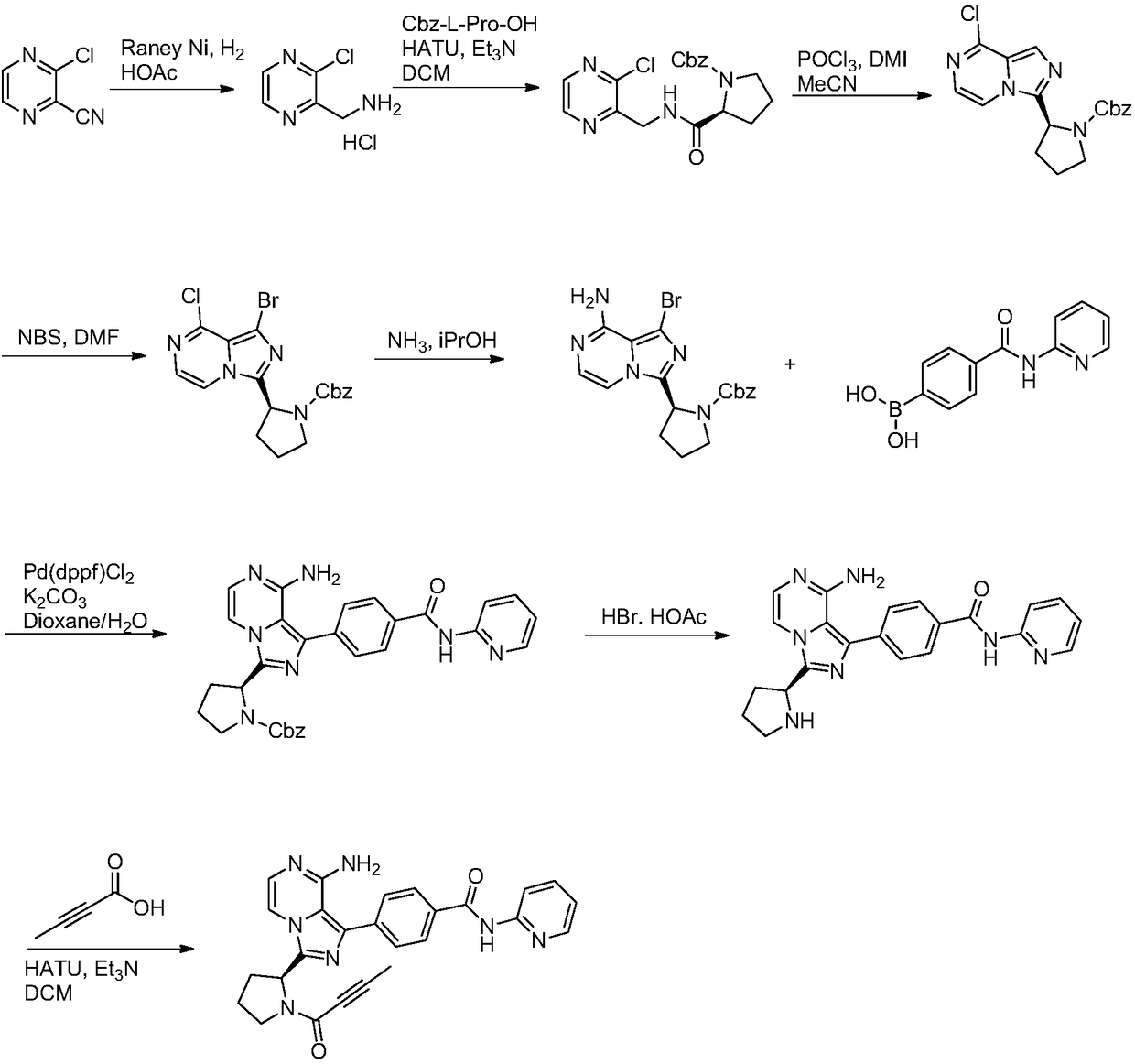
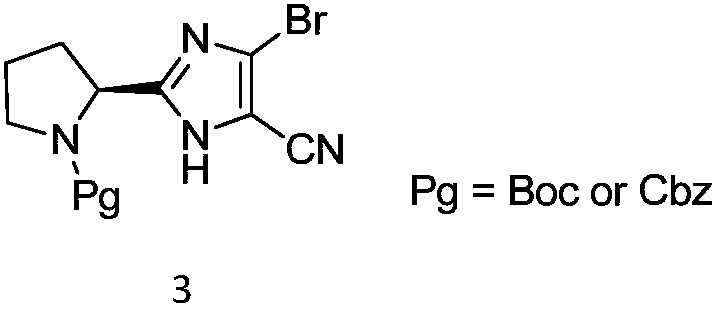
Acalabrutinib and synthesizing method for intermediate thereof
A synthesis method and technology for intermediates, which are applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high cost of enlarged production routes, cumbersome processes, and excessively long linear steps, and achieve the reduction of heavy metal residues, high product purity, and reduced The effect of process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] Add compound 1a (21.33g, 100mmol), 2-chloro-2-formyl acetonitrile (10.35, 100mmol) and acetonitrile (106mL) into the three-necked flask, add triethylamine (20.23g, 200mmol), stir well and heat to reflux React overnight. At the end of the reaction, some isopropanol was spun off, and water (213 mL) was added to precipitate a large amount of solid, which was filtered, and the crude product was slurried with a mixed solvent of isopropanol and petroleum ether, filtered and dried to obtain compound 2a (21.77 g, 83%). MS(ESI)m / z=263.2[M+H] + .
Embodiment 2
[0049]
[0050] Add compound 1b (24.73g, 100mmol), 2-chloro-2-formyl acetonitrile (10.35, 100mmol) and DMAC (124mL) into the three-necked flask, add potassium carbonate (27.64g, 200mmol), stir well and heat to 75~ React overnight at 80°C. After the reaction was completed, water (247 mL) was added, and a large amount of solid precipitated out, which was filtered, and the crude product was slurried with a mixed solvent of isopropanol and petroleum ether, filtered and dried to obtain compound 2b (23.11 g, 78%). MS(ESI)m / z=297.1[M+H] + .
Embodiment 3
[0052]
[0053] Compound 2a (26.23g, 100mmol) and acetonitrile (131mL) were added into a three-neck flask, stirred evenly, NBS (19.58g, 110mmol) was added, and reacted at 25-30°C for 4-6 hours. At the end of the reaction, part of the acetonitrile was spun off, and 5% sodium bisulfite solution (262 mL) was added to precipitate a large amount of solid, which was filtered, and the crude product was slurried with a mixed solvent of ethyl acetate and petroleum ether, filtered and dried to obtain compound 3a (29.68 g, 87%) . MS(ESI)m / z=341.0[M+H] +1 HNMR(400MHz,DMSO-d6)δ12.72(br,1H),4.66-4.85(m,1H),3.18-3.40(m,2H),1.71-2.30(m,4H),1.14-1.45(m, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com