Percutaneous-absorption tulobuterol controlled release patch and preparation method thereof
An adhesive and adhesive technology, applied in the field of tulobuterol patch preparation, can solve the problems of increased blood drug concentration, poor skin permeability, difficulties, etc., and achieve long release time, improved curative effect, and stable release speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
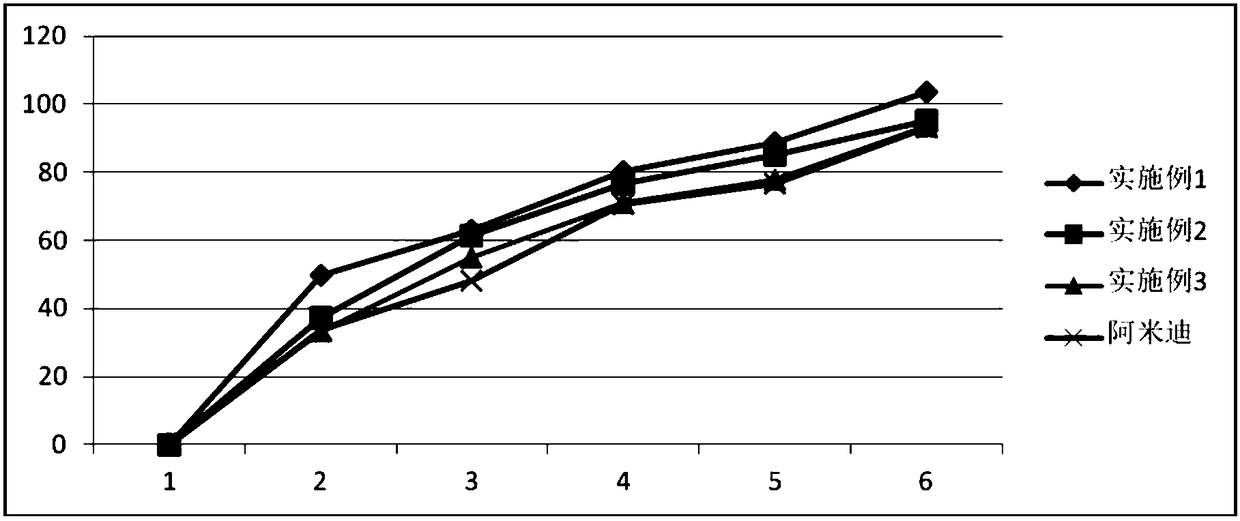
Embodiment 1
[0024] prescription:
[0025]
[0026] experiment procedure
[0027] Take OppanolB 15 SFN, OppanoiB 10 N, polybutene, resin, and tulobuterol in the above-mentioned prescription respectively, add n-hexane to dissolve in a round bottom flask, reflux at 100°C±10°C for 5h, lay a film, and dry naturally .
Embodiment 2
[0029] prescription:
[0030]
[0031] experiment procedure
[0032] Take OppanolB 15 SFN, OppanoiB 10 N, polybutene, resin, and tulobuterol in the above-mentioned prescription respectively, add n-hexane to dissolve in a round-bottomed flask, reflux at 100°C±10°C for 1 hour, lay a film, and dry naturally .
Embodiment 3
[0034] prescription:
[0035]
[0036] experiment procedure
[0037] Take OppanolB 15 SFN, OppanoiB 10 N, polybutene, resin, and tulobuterol in the above-mentioned prescription quantities respectively, add n-hexane to dissolve in a round-bottomed flask, lay a film, and let it dry naturally.
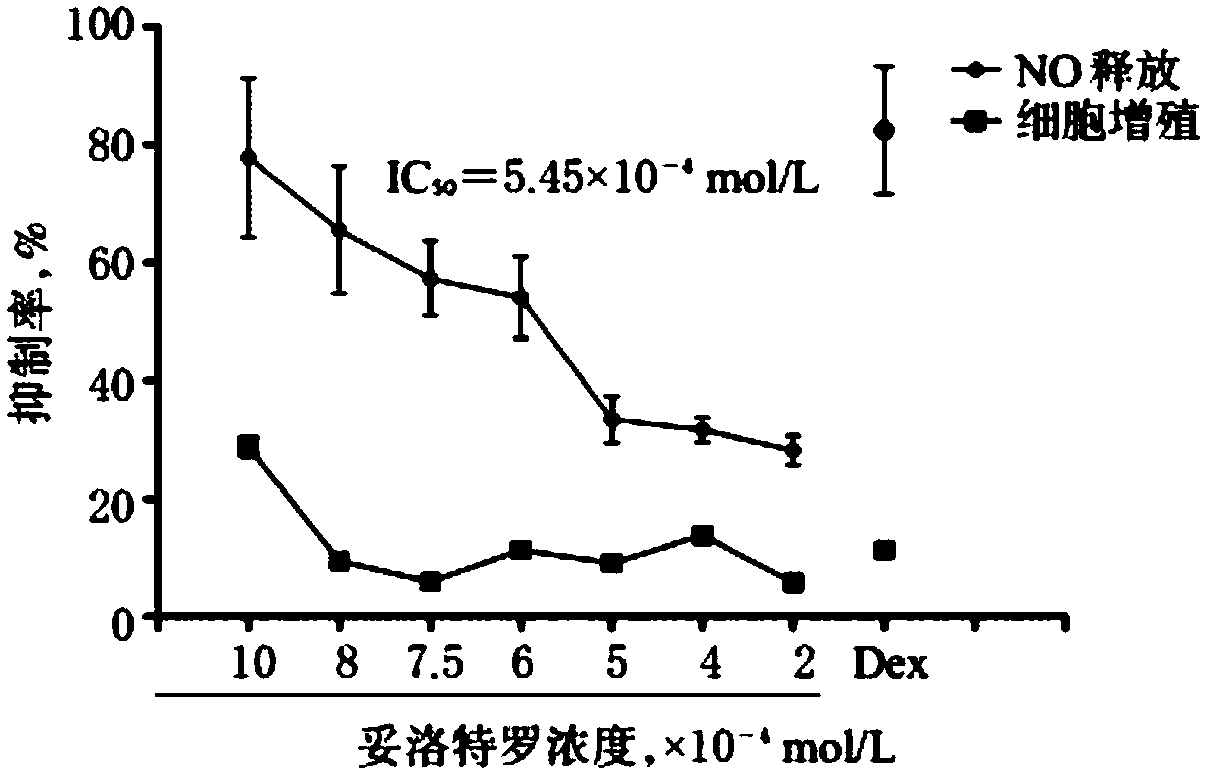
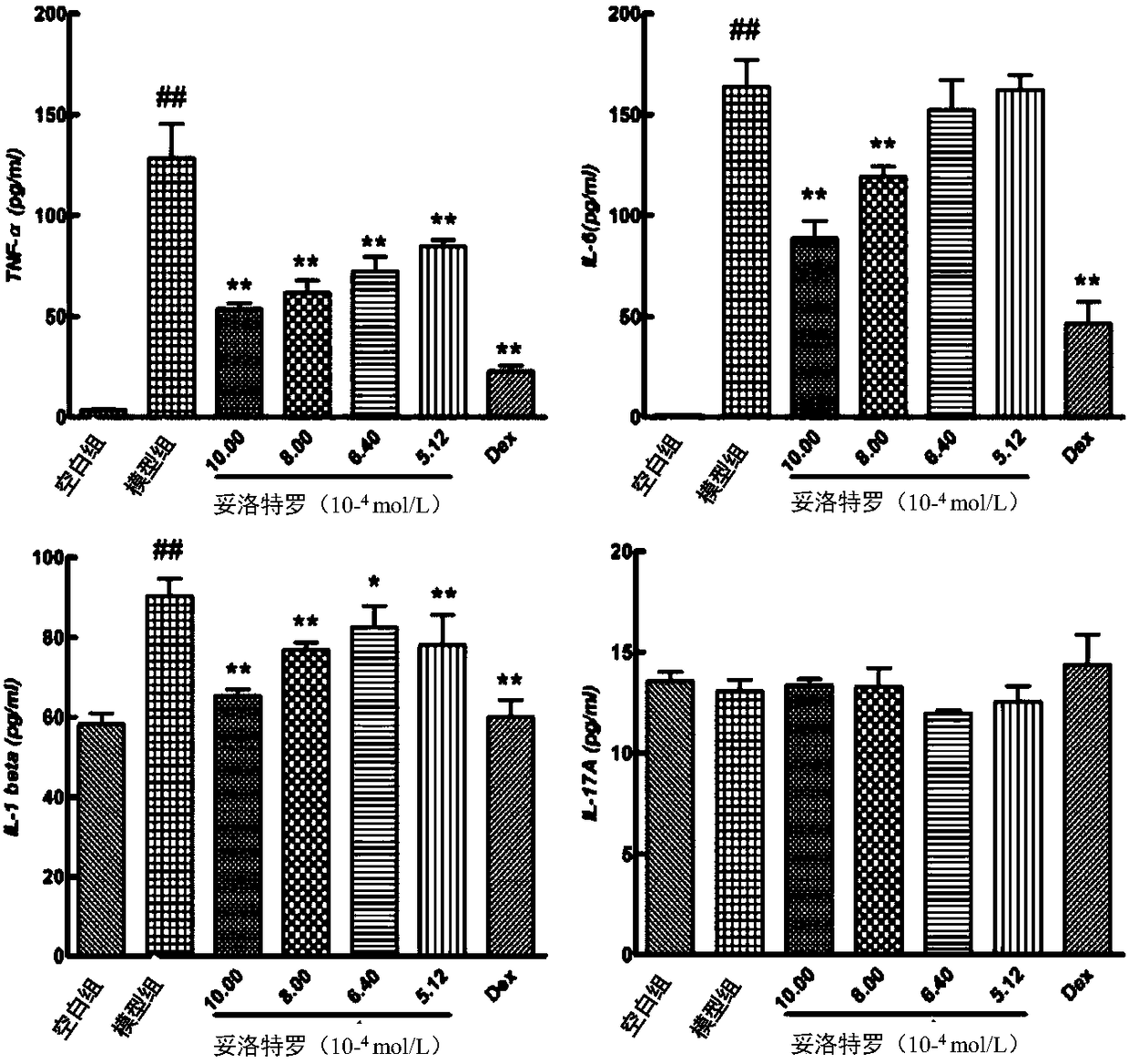
[0038] Pharmacological experiment
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com