Crystal form of bazedoxifene L-lactate
A bazedoxifene and lactate technology, applied in the field of medicinal chemistry, can solve problems such as poor stability, and achieve the effects of good stability, improved water solubility, and avoidance of crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
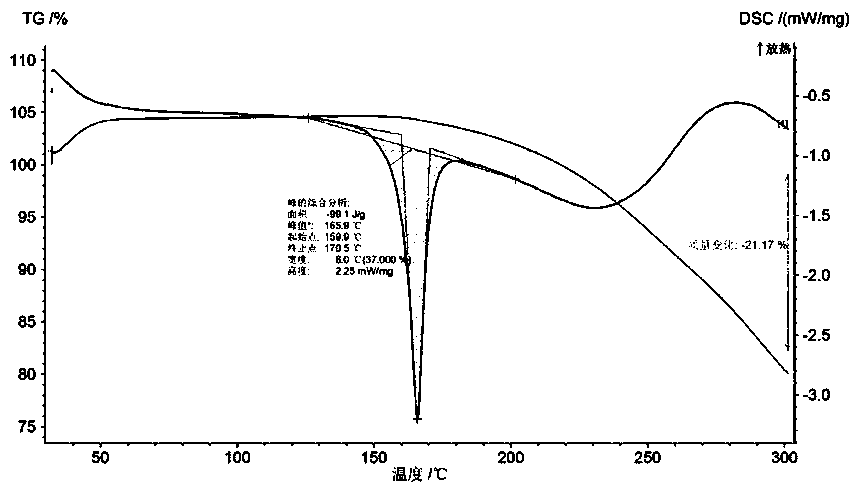
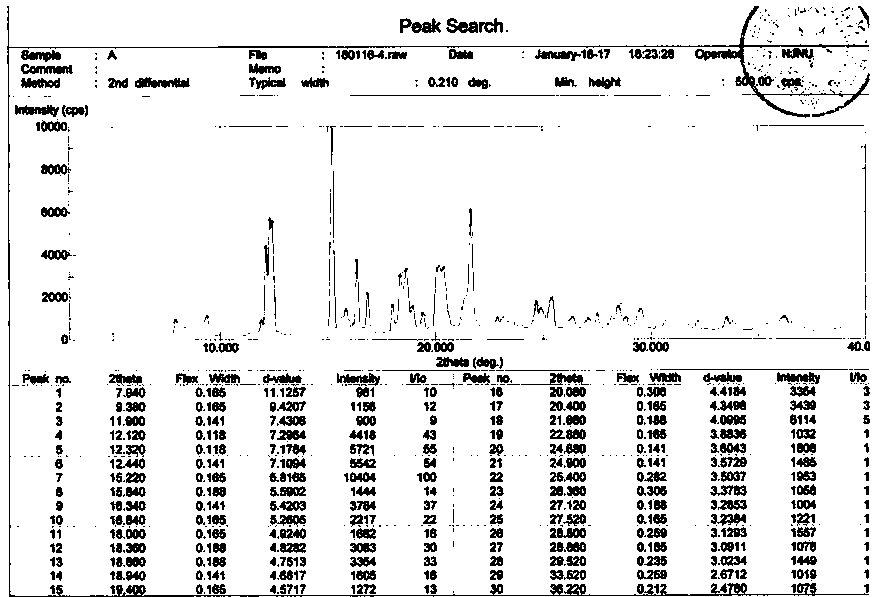
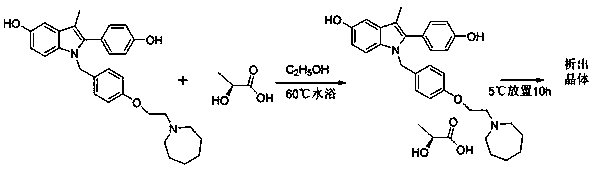
[0022] Dissolve 0.25g of L-lactic acid in 5mL of absolute ethanol at room temperature, heat in a 50ml reaction flask in a water bath at 60°C for 15min, add 1.00g of bazedoxifene and heat in a water bath at 60°C for another 20min, cool naturally, and refrigerate at 5°C After standing for 10 h, after crystallization, suction filtration and drying gave 0.79 g of white crystals with a yield of 66.4%. Characterize the crystal, its MP: 160-171°C, the X-ray powder diffraction pattern exhibited by this crystal form contains peaks with the following spacing values: 9.38 Å, 12.12 Å, 12.32 Å, 12.44 Å, 15.22 Å, 16.34 Å , 16.84 Å, 18.00 Å, 18.36 Å, 18.66 Å, 18.94 Å, 19.40 Å, 21.66 Å. IR: ν3396.32, ν2929.69, ν2961.31, ν2869.35, ν1611.51, ν1509.95, ν1246.44, ν1214.77, ν842.77, ν819.94. 1 HNMR: (500 MHz, CDCl 3 )δ 7.20-7.13 (m, 2H), 7.09-7.03 (d, 1H), 6.90-6.84 (m, 2H), 6.82-6.78 (m, 1H), 6.77-6.71 (m, 4H), 6.61-6.55 (dd, 1H), 5.12-5.08(s, 1H), 4.80-4.75(s, 2H), 4.30-4.25(s, 1H), 3.98-3.9...
Embodiment 2
[0024] Dissolve 0.25g of L-lactic acid in 5ml of isopropanol at room temperature, heat in a 50ml reaction flask in a water bath at 60°C for 15min, add 1.08g of bazedoxifene and heat in a water bath at 60°C for 20min, cool naturally, and refrigerate at 5°C After standing for 10 hours, the crystals were precipitated, filtered by suction, and dried to obtain 0.56 g of white crystals, with a yield of 47.1%. The characterization results are consistent with Example 1.
Embodiment 3
[0026] Dissolve 0.30 g of L-lactic acid in 8 ml of absolute ethanol at room temperature, heat in a 50 ml reaction flask in a water bath at 60°C for 15 minutes, add 1.01 g of bazedoxifene and heat in a water bath at 60°C for 20 minutes, cool naturally, and refrigerate at 5°C After standing for 10 hours, the crystals were precipitated, filtered by suction, and dried to obtain 0.59 g of white crystals, with a yield of 49.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com