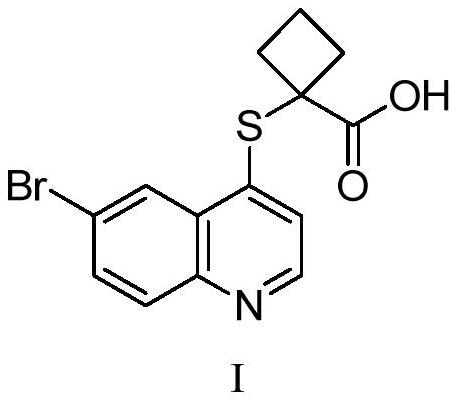
A kind of pharmaceutical composition containing uric acid transport protein inhibitor and preparation method thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of dissolution and the decrease of the dissolution rate of pharmaceutical compositions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
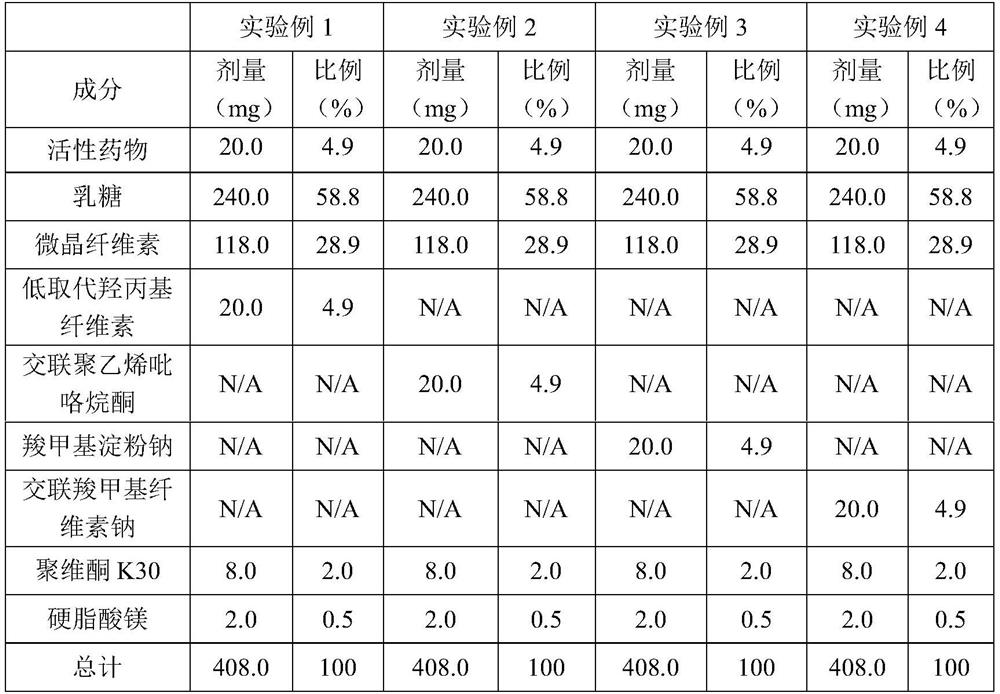
[0049] The active pharmaceutical ingredient 1-((6-bromoquinolin-4-yl)thio)sodium cyclobutanate is mixed with lactose and microcrystalline cellulose, and then respectively mixed with low-substituted hydroxypropyl cellulose and cross-linked polyvinylpyrrolidone , sodium carboxymethyl starch and croscarmellose sodium are mixed, the binder is polyvinylpyrrolidone K30 aqueous solution, the granules are prepared by a high-speed shear granulation process, and then the granules are dried, sieved, and then mixed with stearin Magnesium acid is mixed evenly and pressed into tablets. See Table 1 for specific data.
[0050] Table 1
[0051]
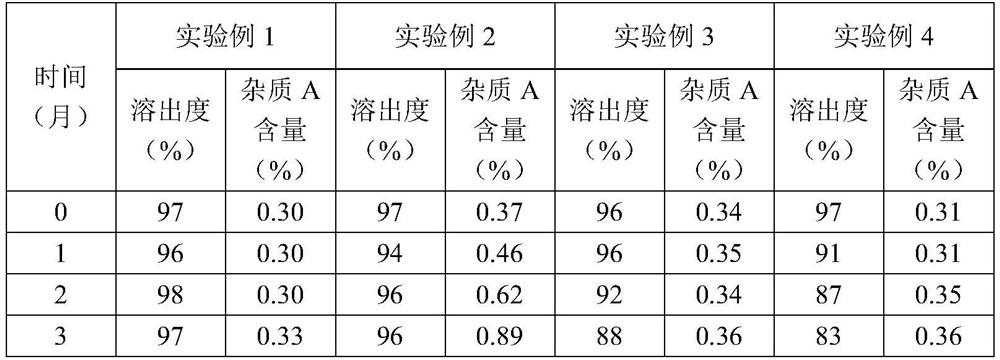
[0052] Put the tablets prepared in Experimental Examples 1 to 4 into aluminum foil bags, place them under the condition of 40°C / RH75%, and take samples at 0, 1, 2, and 3 months to determine the dissolution rate and impurity A content of the tablets in 45 minutes , to investigate the stability of the tablet. The results are shown in Table 2.
[...
Embodiment 2
[0057] The active pharmaceutical ingredient 1-((6-bromoquinolin-4-yl)sulfanyl) sodium cyclobutanate is mixed with mannitol and microcrystalline cellulose, the binder is polyvinylpyrrolidone K30 aqueous solution, and a fluidized bed is used for one step The granulation process produces granules which are then dried and sieved. The prepared granules are respectively mixed with low-substituted hydroxypropyl cellulose and cross-linked polyvinylpyrrolidone, then mixed with magnesium stearate and uniformly pressed into tablets. See Table 3 for the tablet compositions of Experimental Examples 5 to 6.
[0058] table 3
[0059]
[0060] The tablets prepared in Experimental Examples 5 and 6 were put into aluminum foil bags, placed under the condition of 40°C / RH75%, and the 45-minute dissolution rate and related substances of the tablets were sampled and determined in 0 and January, and the properties of the tablets were investigated. stability. The results are shown in Table 4.
...
Embodiment 3
[0065] The active pharmaceutical ingredient 1-((6-bromoquinolin-4-yl)thio)sodium cyclobutanate is mixed with lactose and microcrystalline cellulose, respectively mixed with low-substituted hydroxypropyl cellulose and cross-linked polyvinylpyrrolidone , and then mixed with talcum powder and magnesium stearate evenly and compressed into tablets. See Table 5 for the tablet compositions of Experimental Examples 7 to 8.
[0066] table 5
[0067]
[0068] The tablets prepared in Experimental Examples 7 and 8 were put into aluminum foil bags, placed under the condition of 40°C / RH75%, and samples were taken in 0 and January to determine the dissolution rate and related substances of the tablets in 45 minutes, and the properties of the tablets were investigated. stability. The results are shown in Table 6.
[0069] Table 6
[0070]
[0071] The above results show that during the stability test, when low-substituted hydroxypropyl cellulose is selected for use in the pharmaceut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com