Method for performing aldol condensation reaction in micro-channel reactor
A technology of microchannel reactor and aldol condensation, applied in chemical instruments and methods, organic chemical methods, preparation of organic compounds, etc., can solve the problems of large amount of catalyst usage, difficult control, uneven stirring, etc., and achieve stereoselective Enhanced sex and regioselectivity, avoid exposure to toxic and hazardous substances, and avoid the effect of raw material recycling process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of 2,2-dimethyl-3-hydroxypropionaldehyde: 37% formaldehyde (paraformaldehyde can also be used), 10% sodium hydroxide and isobutyraldehyde are used as raw materials without using other solvents.
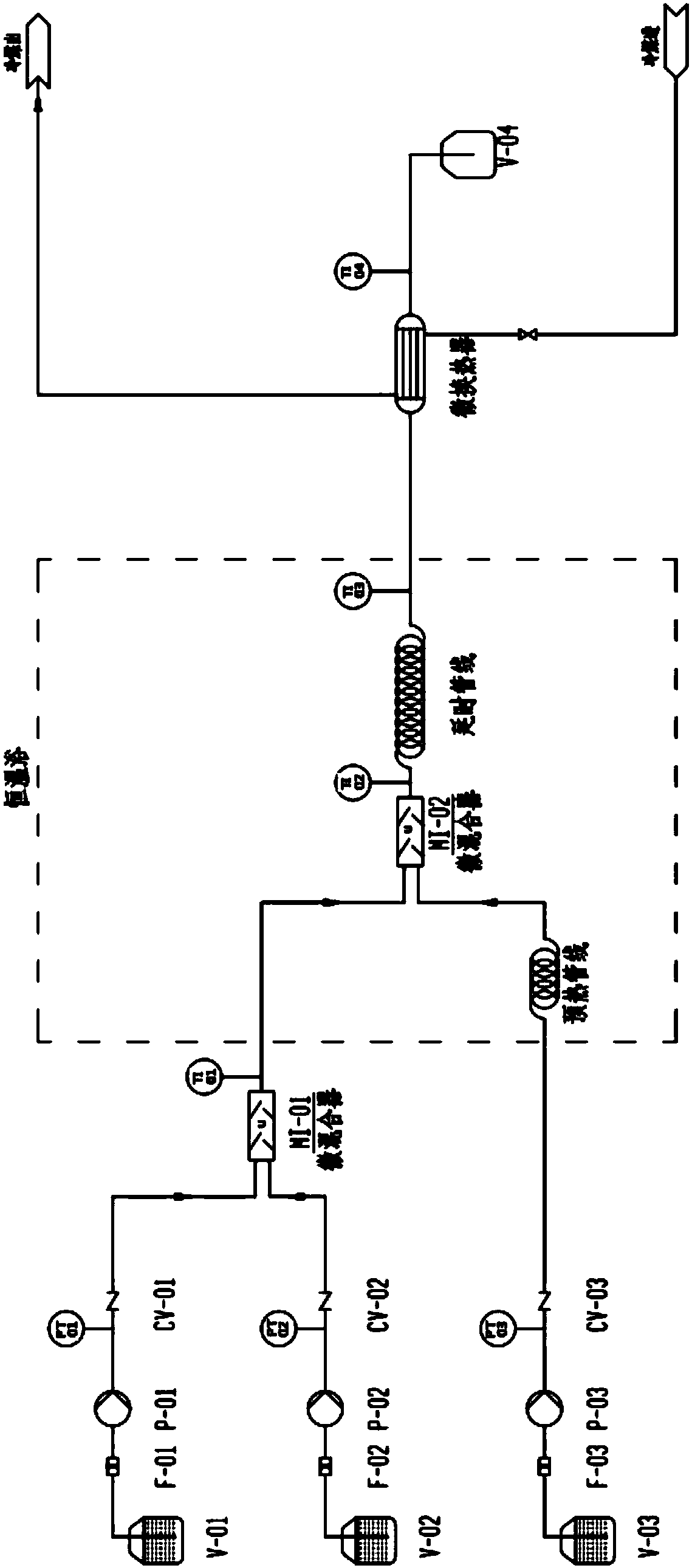
[0044] 1) Connect the microreactor system;
[0045] 2) Raise the temperature of the micro-mixer U and the delay line of the tube reactor to 80°C by a constant temperature bath, and balance and calibrate the system;
[0046] 3) Store formaldehyde with a mass content of 37% in the storage tank V-01, and feed it from the pump P-01 at a volume flow rate of 8.89mL / min;
[0047] 4) Sodium hydroxide with a mass content of 10.0% is placed in the storage tank V-02 for storage, and is fed by the pump P-02 at a volume flow rate of 2.36mL / min;
[0048] 5) Store isobutyraldehyde with a mass content of 99.0% in storage tank V-03, and feed it from pump P-03 at a volume flow rate of 10.80 mL / min;
[0049] 6) Mix in the micro-mixer U, delay the pipeline through the tube reactor, a...
Embodiment 2
[0055] Preparation of 2-heptylene cyclopentanone: 99% cyclopentanone, 99% n-heptanal, and 1.5% sodium hydroxide are used as raw materials without using other solvents.
[0056] 1) operation is with embodiment 1;
[0057] 2) Mix in the micro-mixer U, pass through the tube reactor to delay the pipeline, and complete the aldol condensation reaction in 15 minutes;
[0058] 3) The material after the reaction is completed enters the receiving tank V-04, cooled and stirred, and sent to HPLC for detection, the conversion rate of n-heptanal is 94%, and the reaction selectivity is 92.5%;
[0059] 4) The pH of the reaction solution was adjusted to neutral, and toluene was used as a solvent for extraction, and the extract was carried with water at 110°C until no more water was formed, and then continued to be concentrated to dryness, with a yield of 86.95%;
[0060] 5) Stop the pump and clean the system.
[0061] Preparation of 2-heptylidene cyclopentanone
Embodiment 3
[0063] Preparation of 2-pentylidene cyclopentanone: 99% cyclopentanone, 99% n-valeraldehyde and 1.5% sodium hydroxide are used as raw materials without using solvent.
[0064] 1) operation is with embodiment 1;
[0065] 2) Mix in the micro-mixer U, pass through the tube reactor to delay the pipeline, and complete the aldol condensation reaction in 3 minutes;
[0066] 3) The material after the reaction is completed enters the receiving tank V-04, cooled and stirred, and sent to HPLC for detection. The conversion rate of n-heptanal is 96%, and the reaction selectivity is 96.3%;
[0067] 4) The pH of the reaction solution was adjusted to neutral, and then the reaction solution was concentrated to dryness, with a yield of 92.4%;
[0068] 5) Stop the pump and clean the system.
[0069] Preparation of 2-pentylidene cyclopentanone
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com