Silicon-based triphenylamine derivative, preparation method thereof and application thereof in perovskite solar cell
A silicon-based triphenylamine and solar cell technology, applied in chemical instruments and methods, circuits, photovoltaic power generation, etc., can solve the problems of low voltage of p-i-n devices, increase the cost of device preparation, not suitable for flexible devices, etc. Conducive to migration and transmission, and conducive to stable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Dissolve 4.00 g of 1,4-dibromobenzene in 50 mL of tetrahydrofuran under the protection of nitrogen, cool to -78°C, slowly add 7.41 mL of 2.4 M n-butyllithium into the solution through a syringe, and react 1 Hour. Then 0.65 g of silicon tetrachloride was dissolved in 5 mL of tetrahydrofuran under the protection of nitrogen and added dropwise to the reaction solution. After reacting at low temperature for 1 hour, the temperature was gradually raised to room temperature. After reacting for 12 hours, 5 mL of water was added to the reaction liquid, and then the solvent was spin-dried under reduced pressure. The mixture was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. Then the organic layer was dried and filtered through anhydrous magnesium sulfate to obtain the filtrate, and the filtrate was spin-dried under reduced pressure, and the obtained crude product was subjected to column chromatography with petroleum ether...
Embodiment 2
[0063] 5.00 g of p-bromoaniline, 14.4 g of p-iodoanisole, 0.28 g of CuI, 12.8 g of KOH and 0.26 g of 1,10-phenanthroline were added to a 250 mL two-necked flask, pumped Nitrogen three times, then add 60 mL of anhydrous oxygen-free toluene, stir at 110 °C for 48 hours. After the reaction, cool to room temperature, filter directly with suction, wash the filter residue three times with dichloromethane, and then spin the filtrate to dryness under reduced pressure. Chromatography afforded 6.02 g of yellow solid 3 with a yield of 53.8%.
[0064] 2.00 g of solid 3 was dissolved in 50 mL of tetrahydrofuran under the protection of nitrogen, cooled to -78°C, 2.72 mL of 2.4 M n-butyl lithium was slowly added into the solution through a syringe, and reacted for 1 hour. Then 1.21 g of isopropanol pinacol borate was added dropwise to the reaction solution. After reacting at low temperature for 1 hour, the temperature was gradually raised to room temperature. After reacting for 12 hours, 5...
Embodiment 3
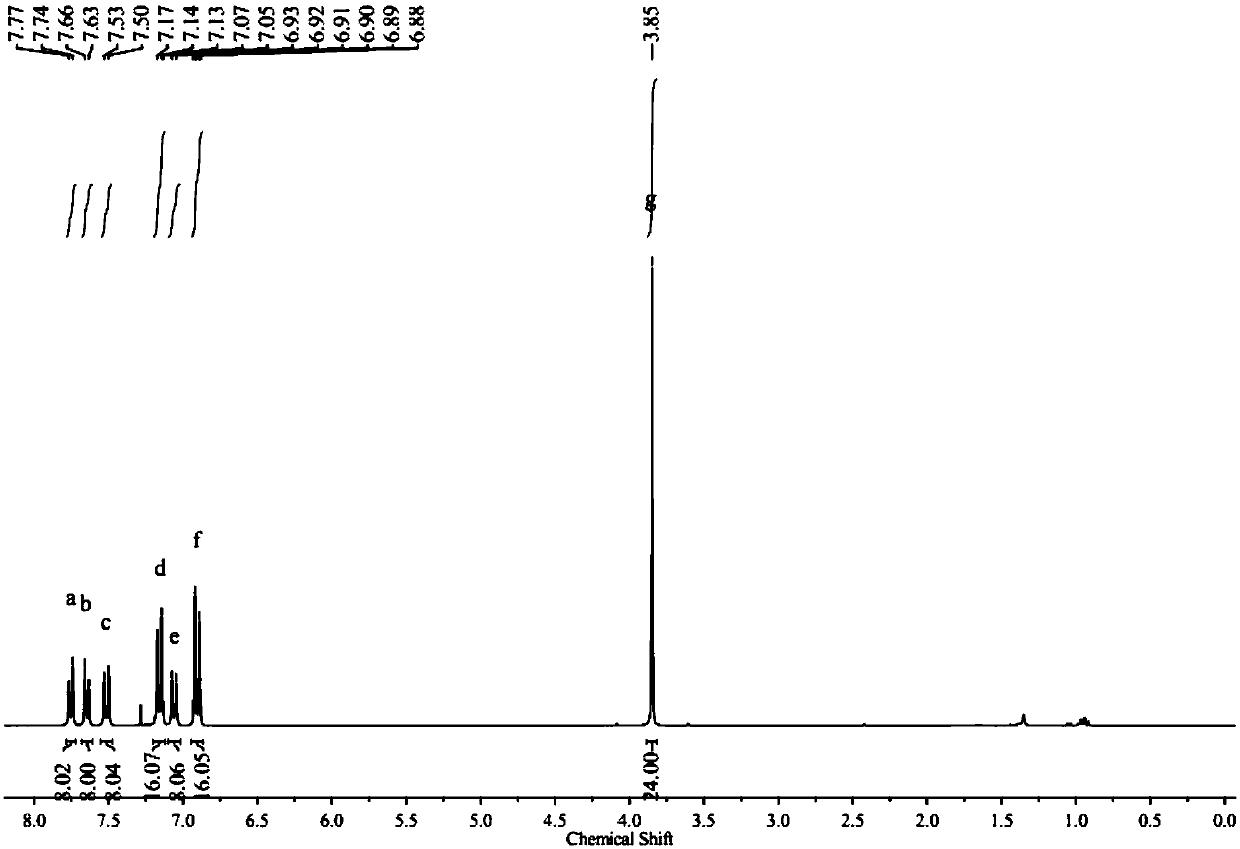
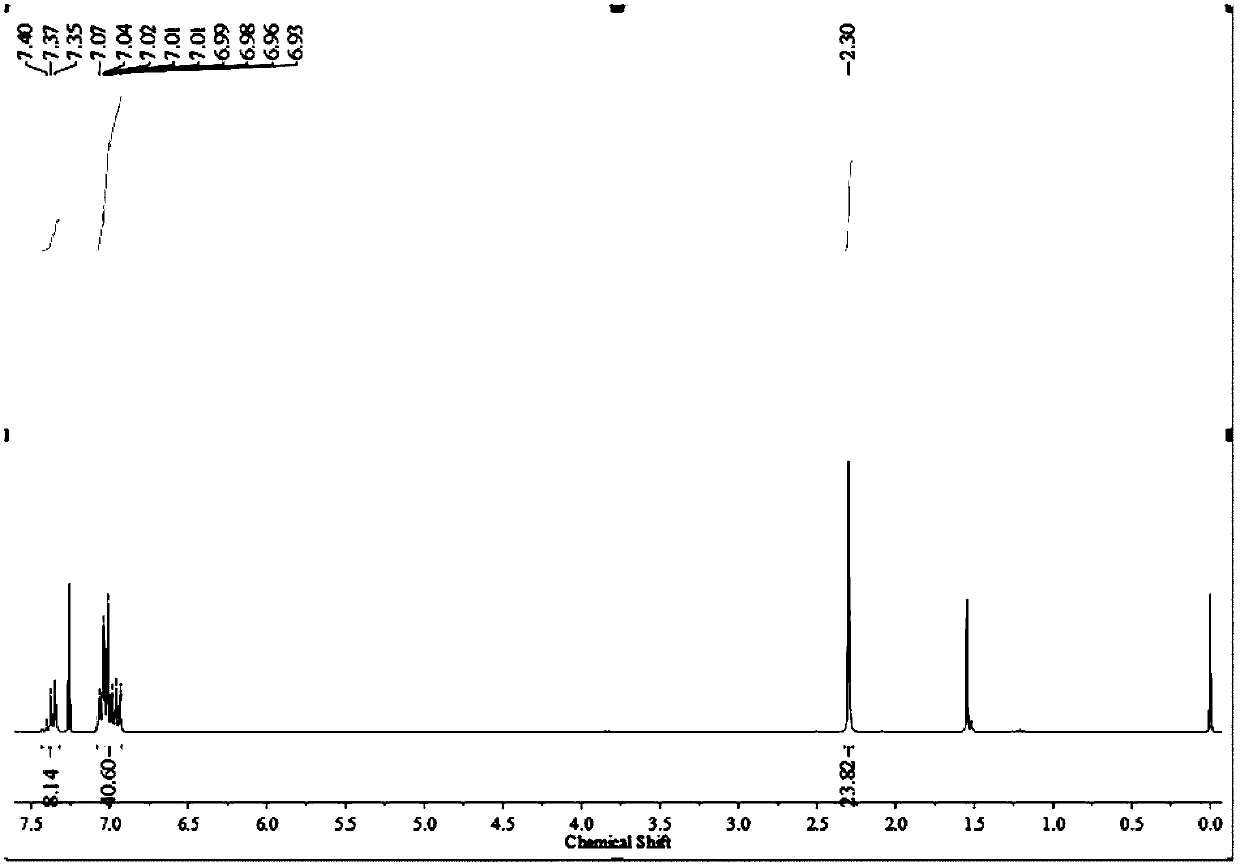
[0068] 0.5 g of white solid 1, 0.685 g of 4,4´-dimethyldiphenylamine, 22 mg of Pd 2 (dba) 3 and 0.44 g of sodium tert-butoxide into a 50 mL reaction flask, pumped nitrogen three times, then added 10 mL of anhydrous oxygen-free toluene and 46 microliters of 1 M tri-tert-butylphosphine toluene solution, at 110 Stir at °C for 48 hours. After the reaction was completed, it was cooled to room temperature, the toluene was distilled off under reduced pressure, the remaining mixture was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. Then the organic layer was dried and filtered through anhydrous magnesium sulfate to obtain the filtrate, and the filtrate was spin-dried under reduced pressure, and the obtained crude product was subjected to column chromatography with petroleum ether and dichloromethane volume ratio 10:1 as the eluent to obtain 0.620 g The white solid C, the yield is 71.9%, image 3 For the silicon-based triphe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com