Salts of aminoquinazoline derivative and application of salts
A diffraction peak and sulfate technology, which can be used in medical preparations containing active ingredients, drug combinations, cardiovascular system diseases, etc., can solve the problems of hindering the binding of EGFR and its inhibitors, increasing the affinity of EGFR and ATP, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] The succinate crystal form A of the compound shown in embodiment 1 formula (I)
[0130] 1. Preparation of title succinate crystal form A
[0131] The free base (0.259g, 0.501mmol) was added to a mixed solution of methanol (2.0mL) and isopropyl acetate (4.0mL), the temperature was raised to 70°C, and acetic acid of succinic acid (0.142g, 1.20mmol) was added dropwise Isopropyl ester solution (1.0 mL), after dropping, the reaction was naturally cooled to room temperature overnight, filtered with suction, the filter cake was washed with a small amount of isopropyl acetate, and dried under vacuum at room temperature to obtain an off-white solid (0.179 g, 56.26%).
[0132] 2. Identification of the title succinate crystal form A
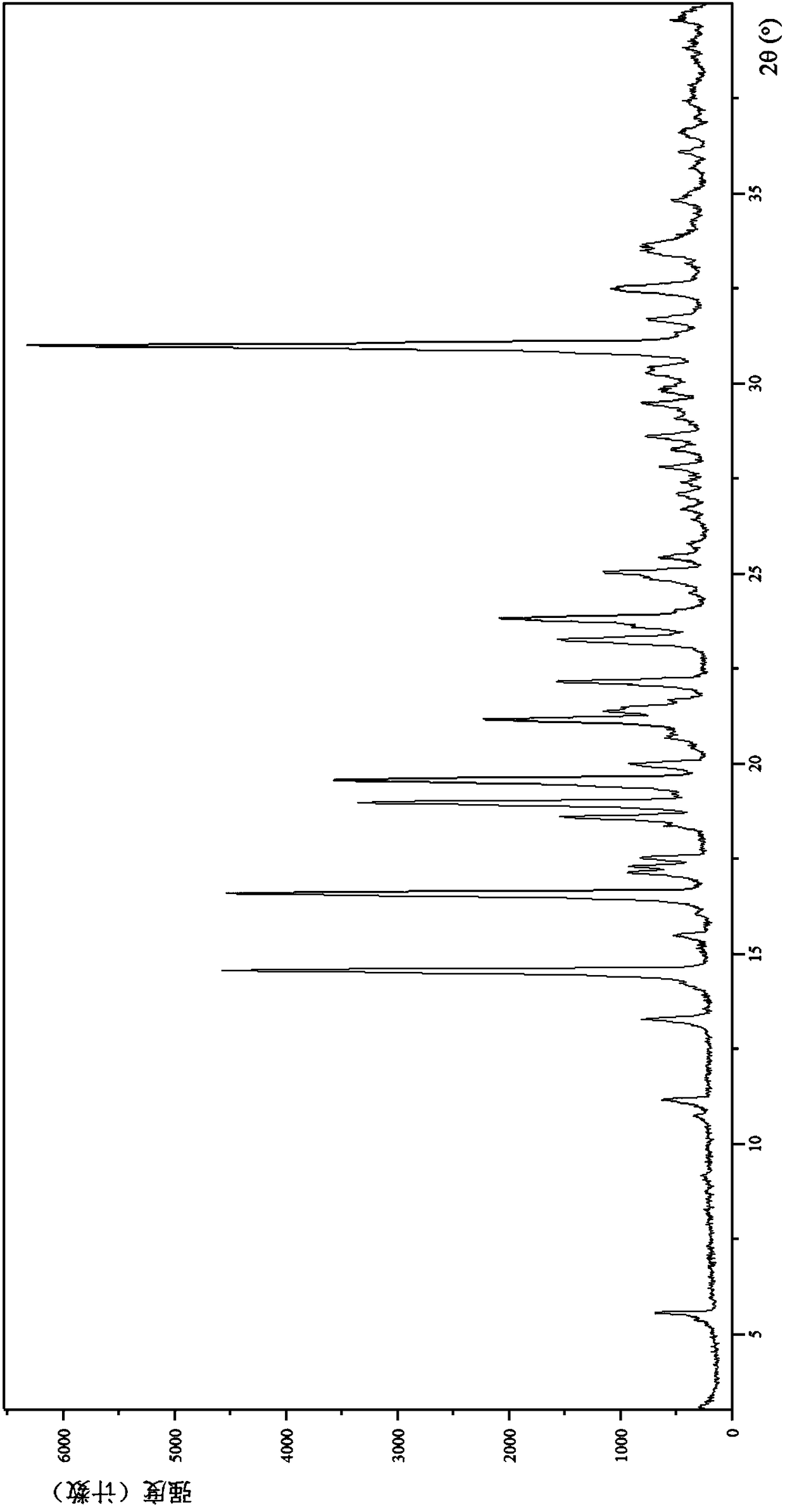
[0133] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 5.52°, 10.70°, 11.11°, 13.24°, 14.51°, 15.44°, 16.04 °,16.54°,17.11°,17....
Embodiment 2
[0135] Embodiment 2 The succinate crystal form B of the compound represented by the formula (I)
[0136] 1. Preparation of title succinate crystal form B
[0137] Add free base (0.516g, 0.998mmol) to ethanol (30.0mL), heat to reflux, filter while hot, continue to reflux the filtrate and add succinic acid (0.248g, 2.10mmol), keep warm for 2.5 hours, and cool the reaction naturally to room temperature, then cooled and crystallized at -15°C, suction filtered, and the filter cake was vacuum-dried at room temperature to obtain a light yellow solid (0.17g, 26.82%).
[0138] 2. Identification of the title succinate crystal form B
[0139] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 5.01°, 7.01°, 9.89°, 10.28°, 11.11°, 13.09°, 13.36 °,13.99°,14.52°,15.11°,15.70°,17.07°,18.58°,18.89°,19.16°,20.20°,20.57°,22.80°,23.29°,23.72°,24.40°,24.52°,25.34°, 26.38°,27.07°...
Embodiment 3
[0141] The succinate amorphous of the compound shown in embodiment 3 formula (I)
[0142] 1. Preparation of the title succinate amorphous
[0143] Add the free base (5.17g, 10.0mmol) to absolute ethanol (180.0mL), heat to reflux to dissolve, filter with suction, continue to reflux the filtrate, and add succinic acid (1.18g, 10.0mmol) in ethanol dropwise to the filtrate The solution (20.0 mL) was incubated for 4 hours, and the above reaction solution was placed in the injection port of a spray dryer, and spray-dried to obtain an off-white solid (3.086 g, 48.6%).
[0144] 2. Identification of the title succinate amorphous
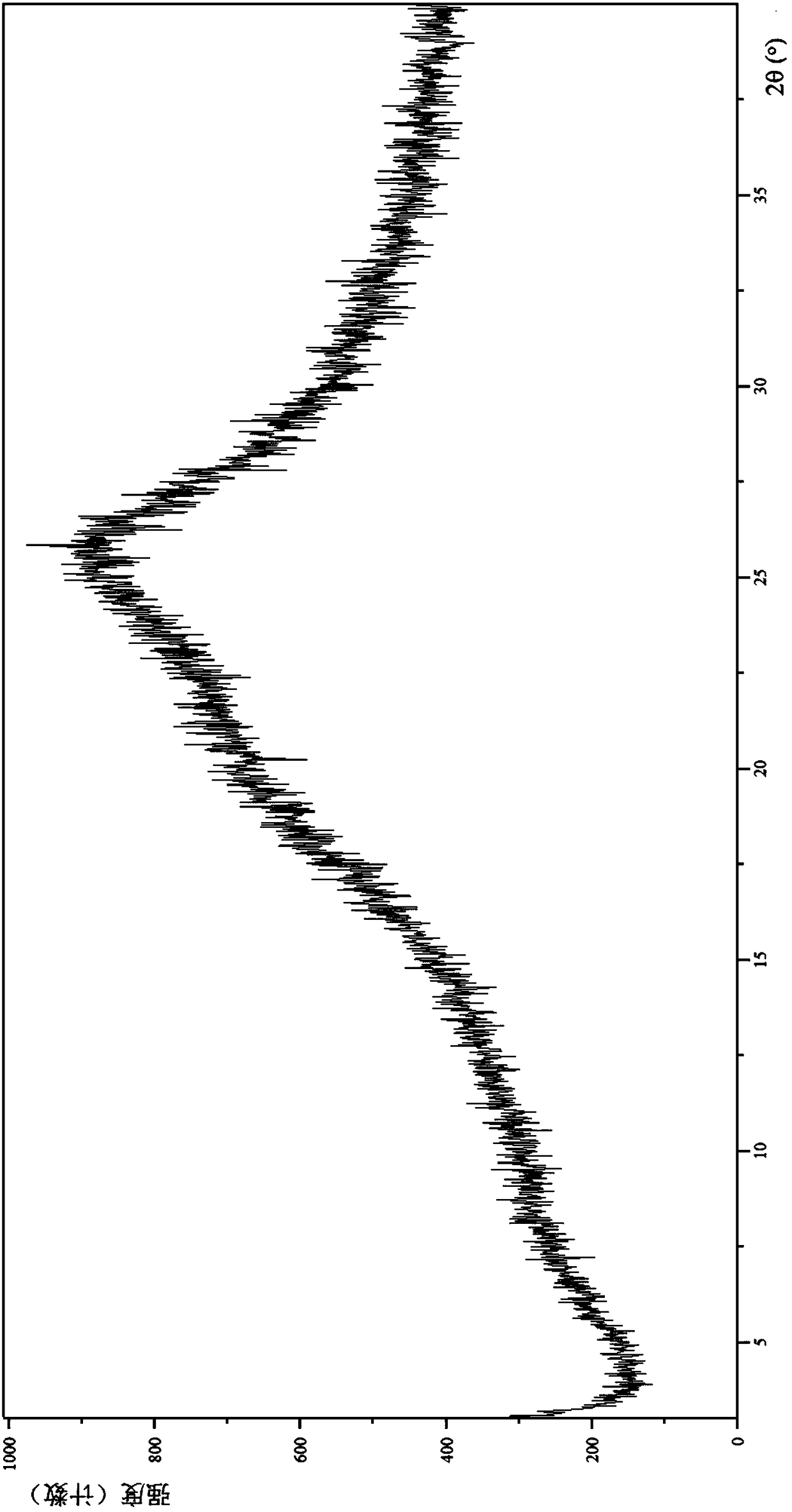
[0145] Through Empyrean X-ray powder diffraction (XRPD) analysis and identification: its X-ray powder diffraction is basically as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com