BRD4 protein inhibitor
A technology of alkyl and compound, applied in the field of medicine, can solve problems such as poor drug metabolism and short patent protection period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
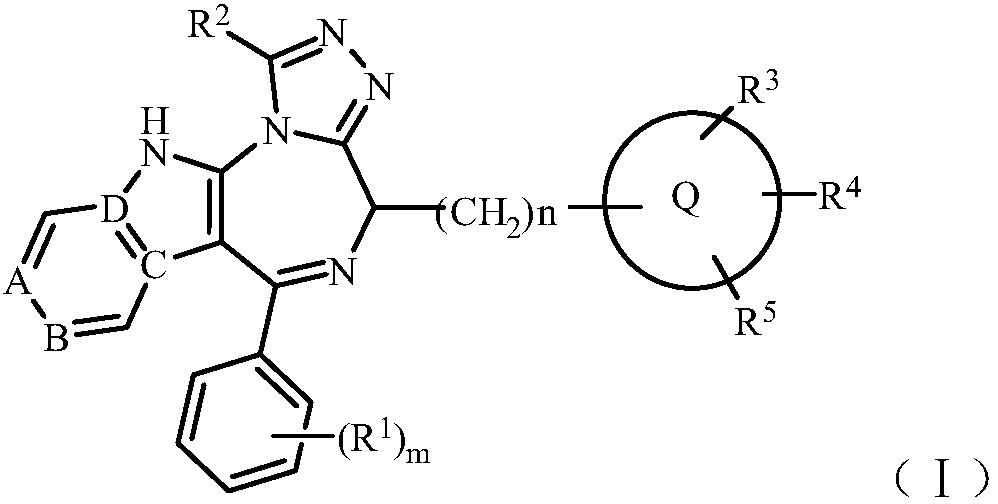
[0086] The preparation method of the compound of the general formula (I) of the present invention comprises making the compound shown in the general formula (IV), its pharmaceutically acceptable salt or its easily hydrolyzed ester, and the compound shown in the general formula (V), its pharmaceutically acceptable Accepted salts, their readily hydrolyzable esters, or their isomers react,
[0087]
[0088] Among them, R 1 , R 2 , R 3 , R 4 , R 5 , A, B, C, D, Q, m and n are as defined above.
[0089] The above-mentioned compounds of the present invention can be synthesized by the methods described in the following schemes and / or other techniques known to those of ordinary skill in the art, but are not limited to the following methods.
[0090] When n is 1, the reaction scheme is:
[0091]
[0092] Reaction steps:
[0093] The preparation of step 1 compound c
[0094] Dissolve raw material a in acetonitrile, add calcium hydroxide, control the temperature below 30°C,...
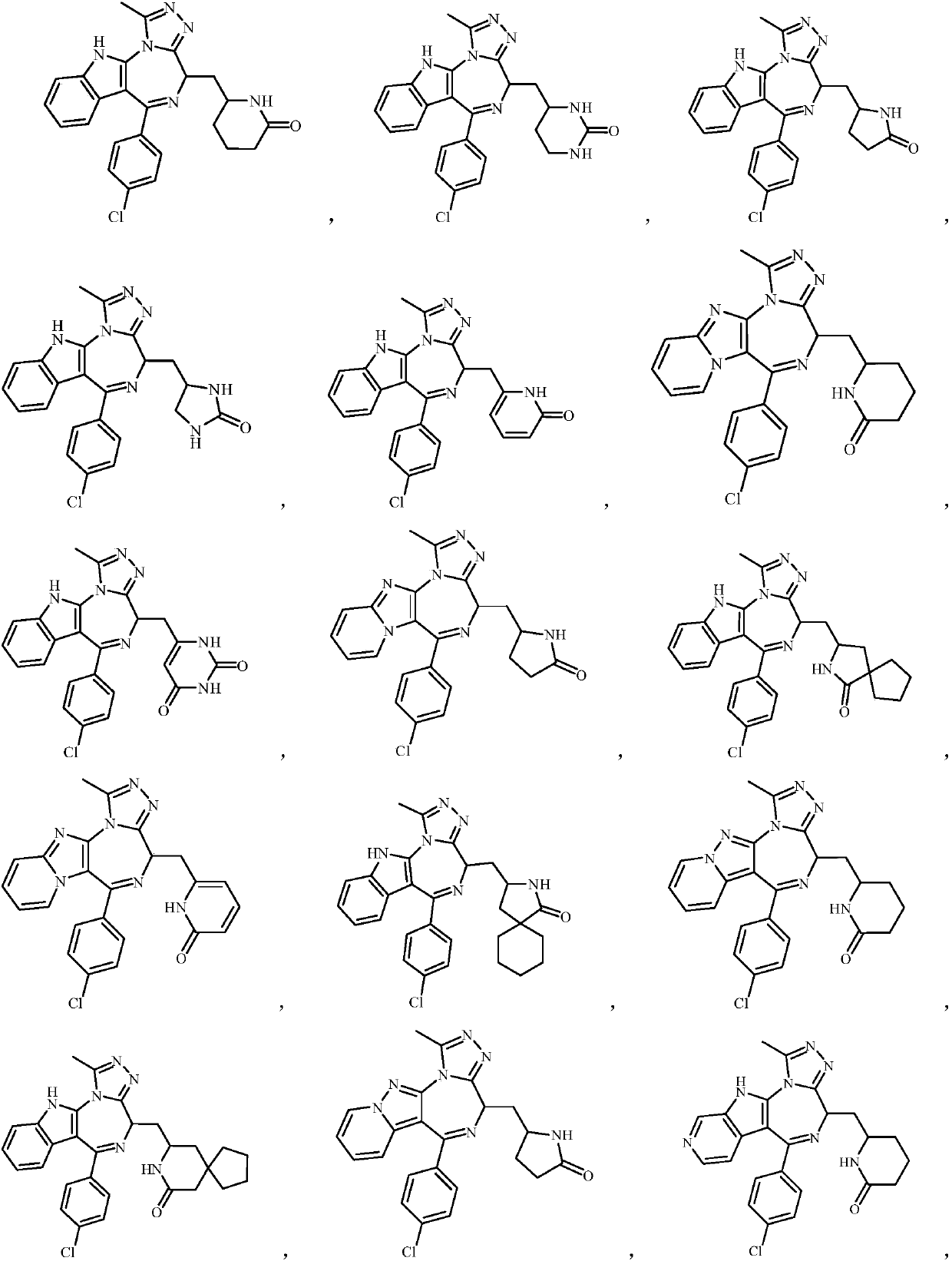
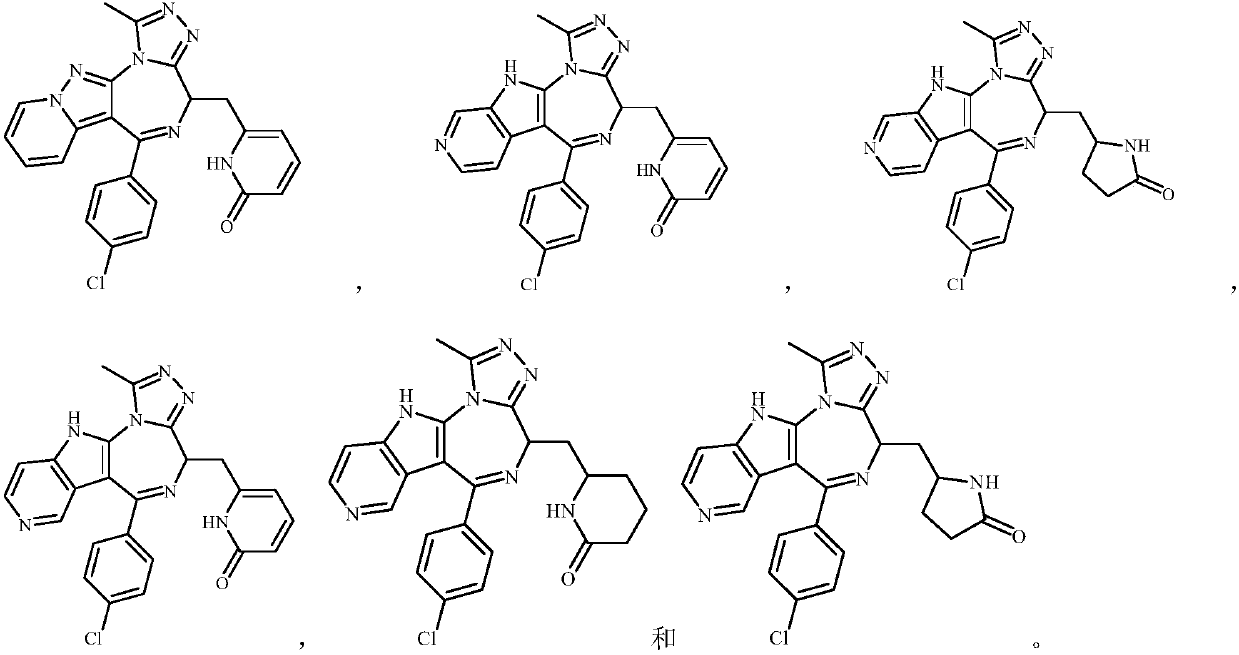
Embodiment 1
[0127] Example 1 6-(((S)-6-(4-chlorophenyl)-1-methyl-4,11-dihydro-[1,2,4]-triazol[3',4':3,4 ] Preparation of [1,4]-diaza[5,6-b]-4-indolyl)methyl)piperidin-2-one (compound 1)
[0128] Step 1: Preparation of (4-chlorophenyl)-(2-hydroxy-1H-3-indolyl)methylketone (Intermediate C)
[0129]
[0130] Dissolve raw material A (133 g, 1.0 mol) in 1 L of acetonitrile, add calcium hydroxide (148 g, 2.0 mol), control the temperature below 30 degrees, add raw material B (210 g, 1.2 mol) dropwise, and then overnight at room temperature. TLC monitored the completion of the reaction. Filtrate, spin the filtrate to dryness, then add 2L of water, extract with EA (500ml*3), wash the organic phase with saturated sodium bicarbonate, dry and spin dry to obtain 210g of a yellow solid with a yield of 77.8%, which is directly used in the next step.
[0131] Step 2: Preparation of (4-chlorophenyl)-(2-chloro-1H-3-indolyl)methylketone (Intermediate D)
[0132]
[0133] Intermediate C (210 g,...
Embodiment 2
[0148] Example 2 6-(((S)-6-(4-chlorophenyl)-1-methyl-4,11-dihydro-[1,2,4]-triazol[3',4': 3,4] Preparation of [1,4]-diaza[5,6-b]-4-indolyl)methyl)hexahydropyrimidin-2-one (compound 2)
[0149] For the synthesis method, refer to Reference Example 1.
[0150] Molecular formula: C 24 h 22 N 7 Molecular weight of ClO: 459.16 LC-MS(M+H) + :460
[0151] 1H NMR(DMSO)δ1.56(2H,t),δ1.70(2H,m),δ2.39-2.57(5H,m),δ3.90(1H,m),δ4.60(1H, t),δ7.32-7.67(2H,m)7.49(2H,s),δ7.79(2H,s),δ7.99-8.12(2H,m),δ9.01(1H,br),δ10 .32(2H,br).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com