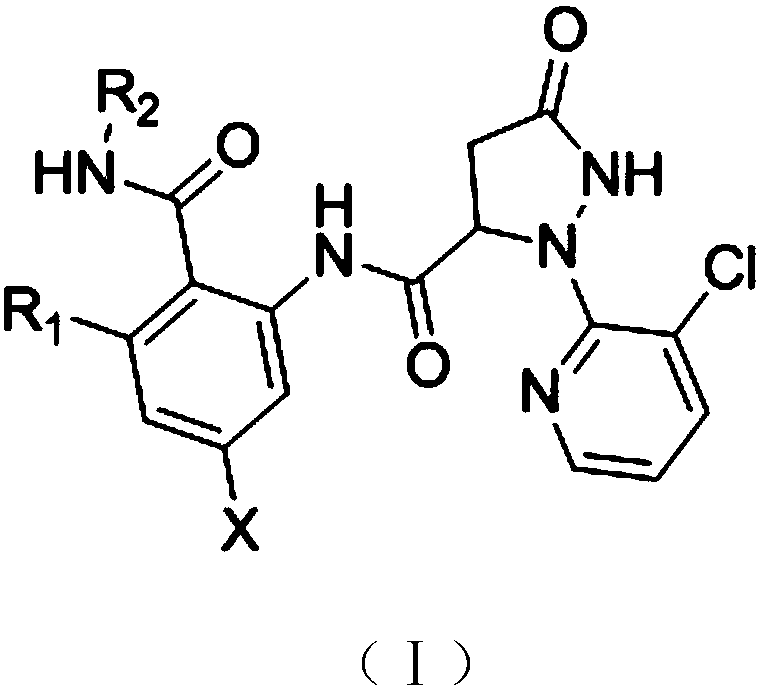
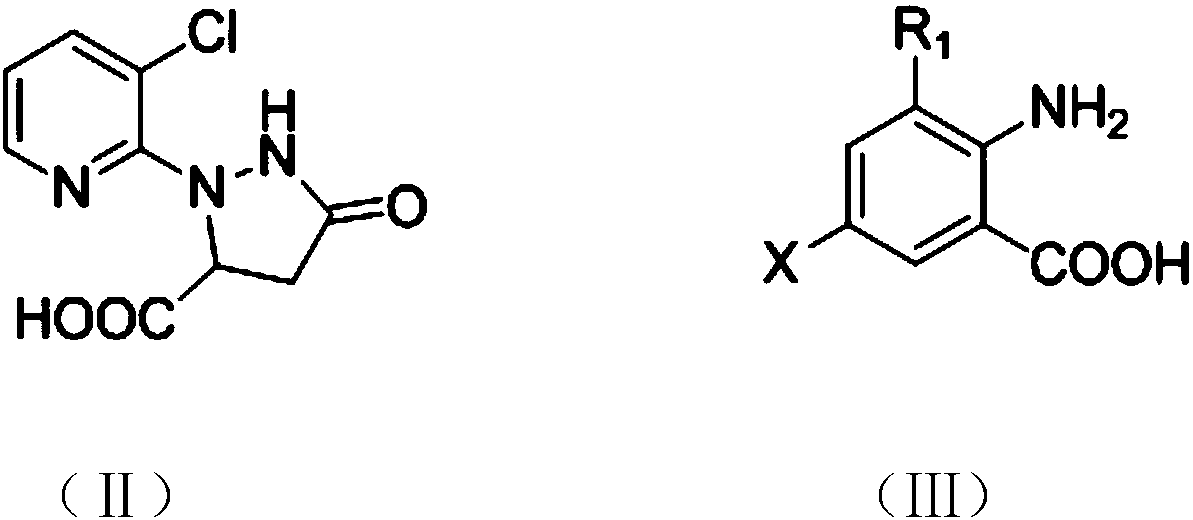
Bisamide compound and synthesizing method and application thereof
A synthesis method and compound technology, applied in the field of synthesis of bisamide compounds, can solve the problem of low activity of homopteran pests, achieve excellent insecticidal activity, simple preparation method, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Step A: Add 20mL of 50% hydrazine hydrate to an ethanol solution containing 20mL of 2,3-dichloropyridine (7.35g, 0.05mol) at room temperature, heat to reflux for 5h, cool to room temperature, and collect the white needle-like solid by filtration Product 3-chloro-2-hydrazinopyridine 6.75g, yield 94.4%;
[0025] Step B: Add 50mL ethanol in the 100mL reaction flask equipped with mechanical stirring, slowly add 1.2g metal sodium, reflux reaction makes sodium ethylate solution, add 3-chloro-2-hydrazinopyridine ( 6.75 g, 0.05mol), diethyl maleate (9m L, 0.055mol), continue to reflux for 30min, then cool down, filter to obtain a light yellow solid 3-hydroxyl-1-(3-chloropyridin-2-yl)- 1H-pyrazole-5-carboxylic acid ethyl ester (6.48g, 0.024mol), yield 52%;
[0026] Step C: 0.5g sodium hydroxide and 10mL methanol were added to 3-hydroxy-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid ethyl ester (2.7g, 0.01mol ) in a 100mL reaction flask, stirred at room temperature for ...
Embodiment 2-4
[0030] 6-Chloro-2-(3-hydroxy-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-4H-3,1-benzoxazine-4 -Reaction of ketones with different amines, the specific steps are the same as in Example 1, and the new compounds obtained by the reaction are shown in Table 1, and their characterization data are shown in Table 2.
Embodiment 5
[0032] Step A: Add 20mL of 50% hydrazine hydrate to an ethanol solution containing 20mL of 2,3-dichloropyridine (7.35g, 0.05mol) at room temperature, heat to reflux for 5 hours, cool to room temperature, and collect white needles by filtration Solid product 3-chloro-2-hydrazinopyridine 6.75g, yield 94.4%;
[0033] Step B: add 50mL ethanol in the 100mL reaction bottle that mechanical stirring is housed, slowly add 1.2g metallic sodium, reflux reaction makes sodium ethylate solution, add 3-chloro-2-hydrazinopyridine ( 6.75g, 0.05mol), diethyl maleate (9m L, 0.055mol), continue to reflux for 30min, then cool down, filter to obtain light yellow solid 3-hydroxyl-1-(3-chloropyridin-2-yl)- 1H-pyrazole-5-carboxylic acid ethyl ester (6.48g, 0.024 mol), yield 52%;
[0034] Step C: 0.5g sodium hydroxide and 10mL methanol were added to 3-hydroxy-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid ethyl ester (2.7g, 0.01mol ) in a 100mL reaction flask, stirred at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com