Preparation method of dimethyl furan-2, 5-dicarboxylate
A technology of dimethyl furandicarboxylate and hydroxymethyl furfural, applied in directions such as organic chemistry, to achieve the effects of excellent performance, efficient catalyst and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
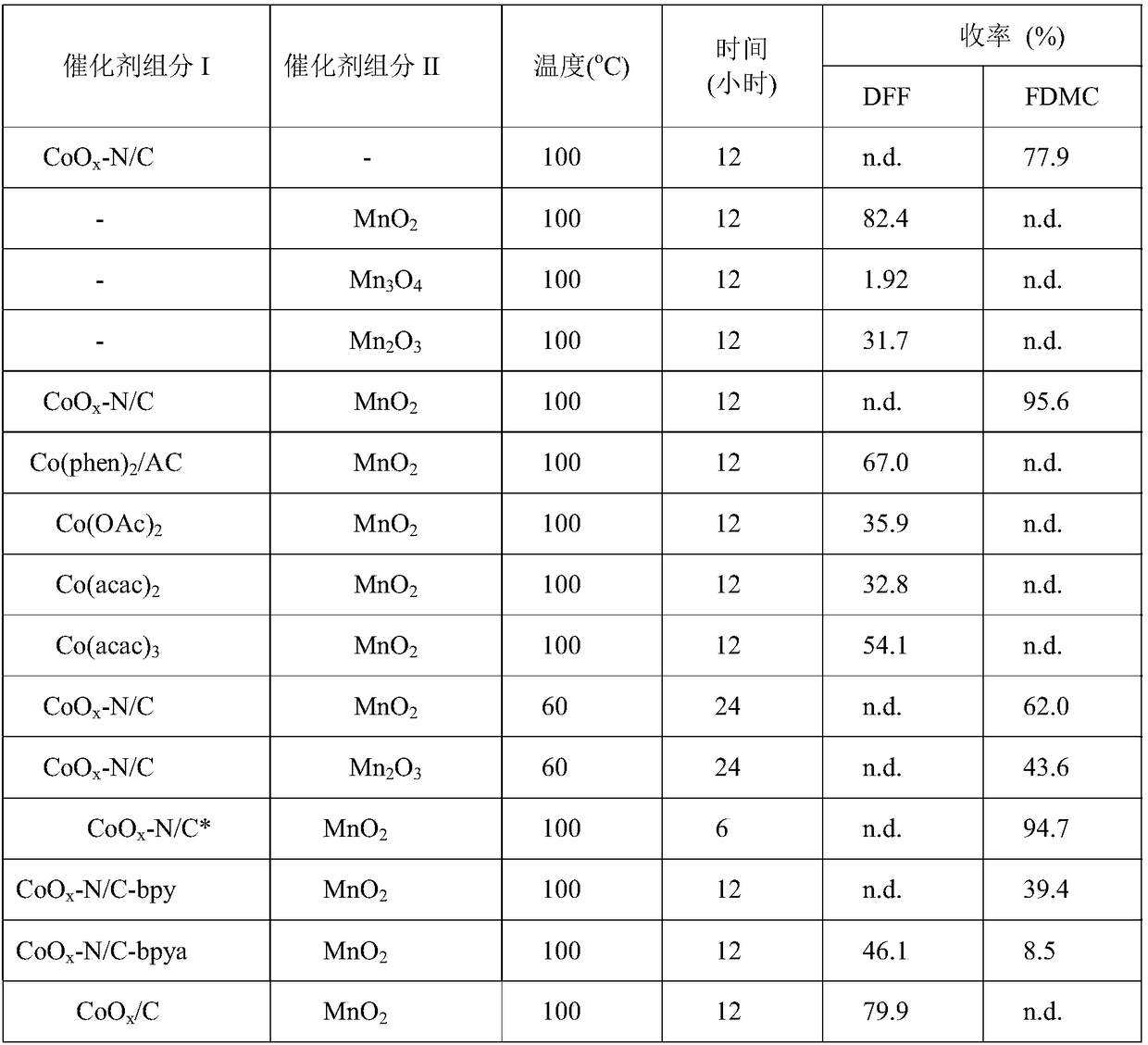
[0015] CoO x -N / C-phen (Co 3.0wt%) catalyst, 0.5mmol 5-hydroxymethylfurfural, and 10ml of methanol are added to a stainless steel high-pressure reaction kettle, which is lined with polytetrafluoroethylene, and the metal: 5-hydroxymethylfurfural =0.13:1 (mol:mol). The temperature was raised to a reaction temperature of 100° C. using an automatic temperature controller, and 0.6 MPa oxygen was added to react for 12 hours, keeping the pressure constant during the reaction. The reaction products were analyzed by GC, and the reaction results are shown in Table 1.
Embodiment 2
[0017] 40mg MnO 2 , 0.5 mmol of 5-hydroxymethylfurfural and 10 ml of methanol were added to a stainless steel autoclave with a polytetrafluoroethylene lining inside. The temperature was raised to a reaction temperature of 100° C. using an automatic temperature controller, and 0.6 MPa oxygen was added to react for 12 hours, keeping the pressure constant during the reaction. The reaction products were analyzed by GC, and the reaction results are shown in Table 1.
Embodiment 3
[0019] 40mg Mn 3 o 4 , 0.5 mmol of 5-hydroxymethylfurfural and 10 ml of methanol were added to a stainless steel autoclave with a polytetrafluoroethylene lining inside. The temperature was raised to a reaction temperature of 100° C. using an automatic temperature controller, and 0.6 MPa oxygen was added to react for 12 hours, keeping the pressure constant during the reaction. The reaction products were analyzed by GC, and the reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com