Compositions and methods for inhibiting gene expression of factor XII
A technology of inhibitory factors and compositions, applied in biochemical equipment and methods, non-effective ingredients of polymer compounds, drug combinations, etc., can solve problems such as the impact of bleeding on life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Example 1. RNAi initiator synthesis.
[0245] A) Synthesis. RNAi initiator molecules were synthesized following the phosphoramidite technique on solid phase for oligonucleotide synthesis. Depending on scale, MerMade96E (Bioautomation) or MerMade12 (Bioautomation) was used. In controlled pore glass (CPG, or Synthesis was performed on solid supports from Prime Synthesis, Aston, PA, USA. All DNA, 2'-modified RNA, and UNA phosphoramidites were purchased from Thermo Fisher Scientific (Milwaukee, WI, USA). Specifically, the following 2'-O-methylphosphoramidite was used: (5'-O-dimethoxytrityl-N 6 -(Benzoyl)-2′-O-methyl-adenosine-3′-O-(2-cyanoethyl-N,N-diisopropylamino)phosphoramidite, 5′-O- Dimethoxytrityl-N 4 -(acetyl)-2′-O-methyl-cytidine-3′-O-(2-cyanoethyl-N,N-diisopropylamino)phosphoramidite, (5′-O- Dimethoxy-trityl-N 2 -(isobutyryl)-2′-O-methyl-guanosine-3′-O-(2-cyanoethyl-N,N-diisopropylamino)phosphoramidite and 5′-O- Dimethoxy-trityl-2'-O-methyl-uridine-3'-O...
Embodiment 2
[0249] Example 2. Melittin-like peptide (MLP) delivery polymers.
[0250] A) Melittin-like peptide (MLP) synthesis. All MLPs were prepared using peptide synthesis techniques standard in the art. Independently of the L or D form, the MLP sequence can be retro.
[0251] B) CDM-NAG (N-acetylgalactosamine) synthesis To a solution of CDM (300 mg, 0.16 mmol) in 50 mL of dichloromethane was added oxalyl chloride (2 g, 10 weight equivalents) and dimethylformamide (5 μL) . The reaction was carried out overnight, after which excess oxalyl chloride and dichloromethane were removed by rotary evaporation to yield CDM acid chloride. The acid chloride was dissolved in 1 mL of dichloromethane. To this solution was added 1.1 molar equivalents of (aminoethoxy)ethoxy-2-(acetylamino)-2-deoxy-β-D-galactopyranoside (i.e., aminodiethoxy-ethyl NAG ) and pyridine (200 μL, 1.5 equiv) in 10 mL of dichloromethane. It was then stirred for 1.5 hours. The solvent was then removed and the resulting so...
Embodiment 3
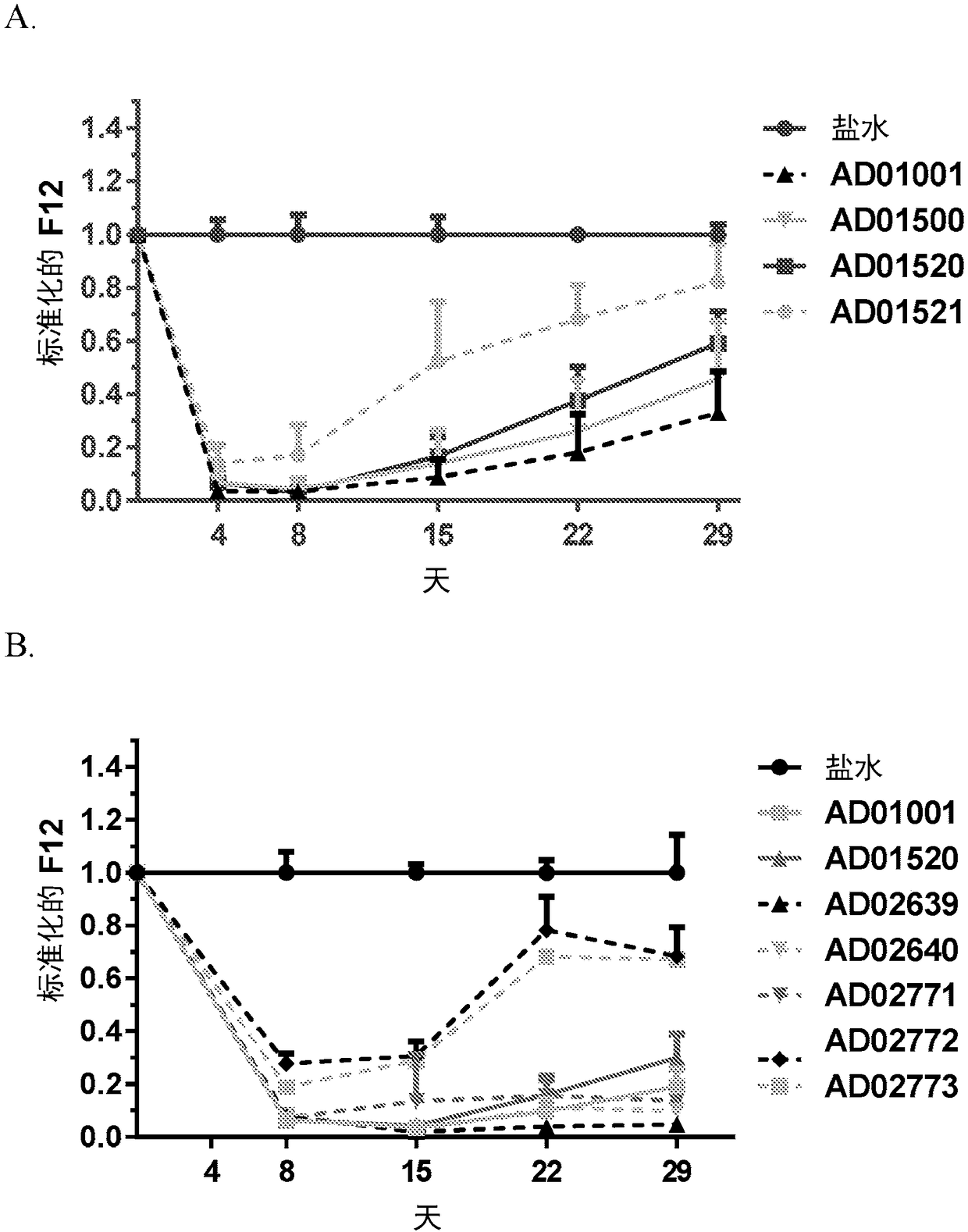
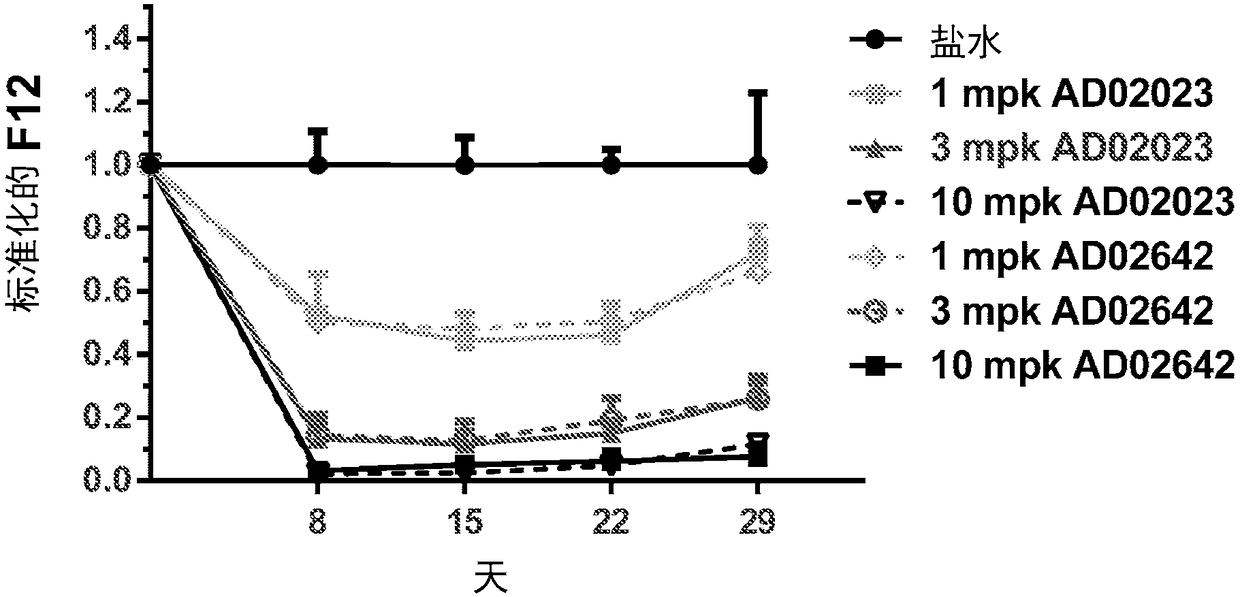
[0270] Example 3. In vitro screening of F12 RNAi initiators.
[0271] A) Human cell background. Candidate sequences identified by in silico analysis as being cross-reactive in humans, nonhuman primates, and mice were screened in vitro as chemically modified canonical siRNAs. Thirty-two in silico identified potential F12 RNAi initiators were synthesized as canonical siRNAs and screened for efficacy in vitro. For screening purposes, the human F12 cDNA sequence (accession number NM_000505) was synthesized and cloned (DNA 2.0, Menlo Park, CA) into a commercially available reporter-based screening plasmid, psiCHECK2 (Promega Corporation, Madison, WI). (Promega)), which produces Renilla luciferase / F12 fusion mRNA. For the efficacy of siRNA in a human background, Hep3B cells, a human hepatocellular carcinoma cell line, were seeded at approximately 10,000 cells / well in 96-well plates. Each of the 32 F12 siRNAs was co-transfected at two concentrations (1 nM and 0.1 nM) with 25 ng F1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com