Composition of pranlukast-containing solid preparation with improved bioavailability and method for preparing same
A composition and drug technology, applied in the directions of non-active ingredients medical preparations, drug combinations, heterocyclic compound active ingredients, etc., can solve problems such as increased preparation cost, and achieve the effects of improved bioavailability and improved release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0057] [Example 1~10] Preparation of prankast wet granules
[0058] The active ingredient (Pranlukast) and diluent were placed in a high speed mixer and mixed. The binder and the surfactant are stirred and dissolved in the solvent to obtain a binder solution. The binding solution was put into the mixture and kneaded in a high speed mixer. A granulation process using a sieve may be added as needed, and the solvent is evaporated using a tray drier. The dried material was sieved with a sieve to obtain wet granules of prankast. Its specific composition is shown in Table 1 below.
[0059] [Table 1]
[0060]
[0061]
Embodiment 11~17
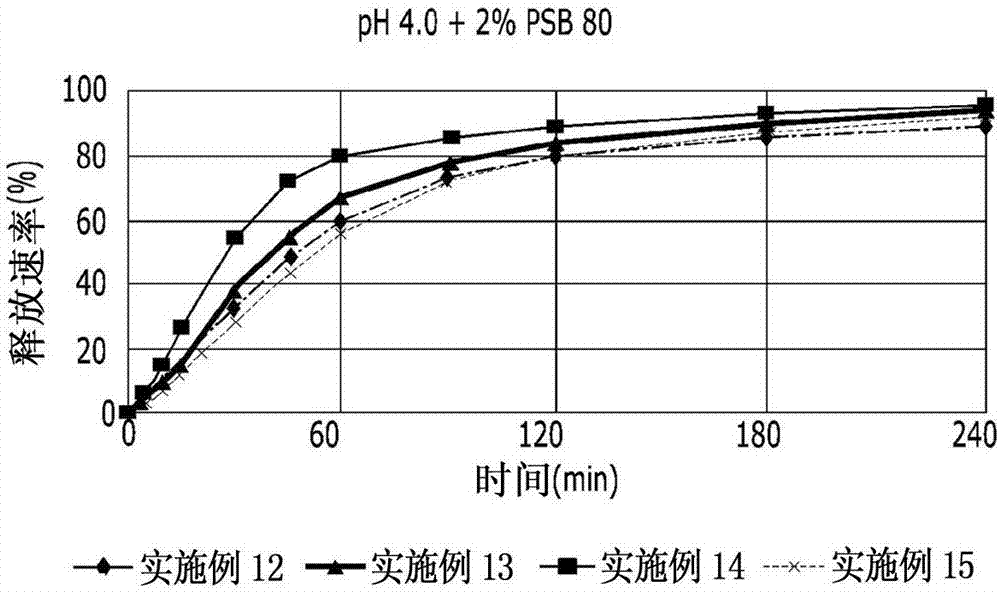
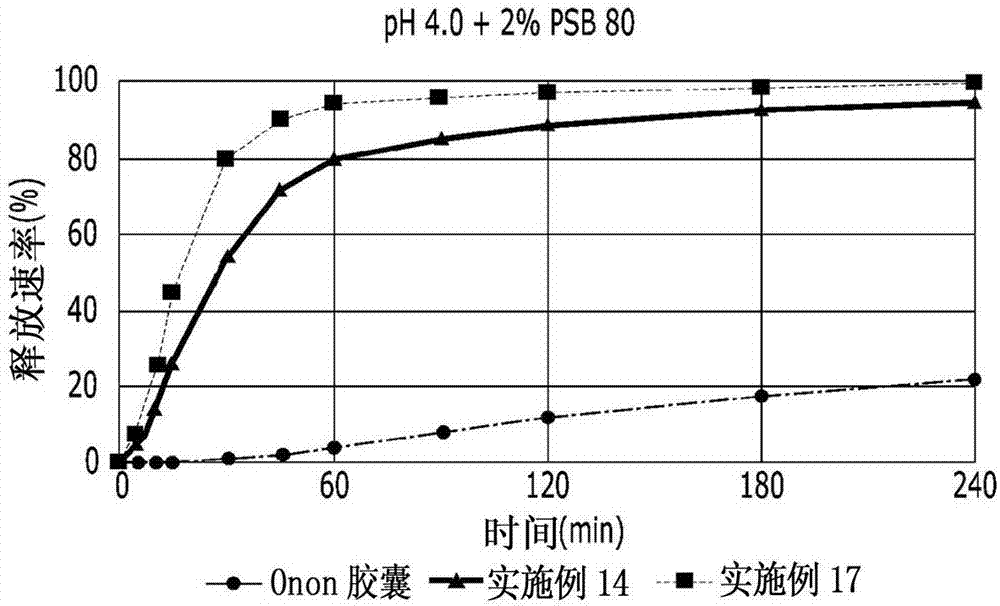
[0062] [Examples 11-17] Preparation of Prankast-containing Tablets
[0063] Tablets were prepared with the ingredients shown in Table 2 below by using the granules prepared in Examples 1, 6, 8 and 10. Specifically, wet granules and disintegrant are put into a mixer and mixed, and lubricant is put and further mixed. After that, Examples 11 to 17 were prepared by performing tablet compression and coating. Examples 11 to 16 were prepared so that each tablet contained 70 mg of pranlukast, and Example 17 was prepared so that each tablet contained 50 mg of pranlukast.
[0064] [Table 2]
[0065]
[0066]
experiment experiment example 1
[0067] [Experimental example 1] comparative dissolution test
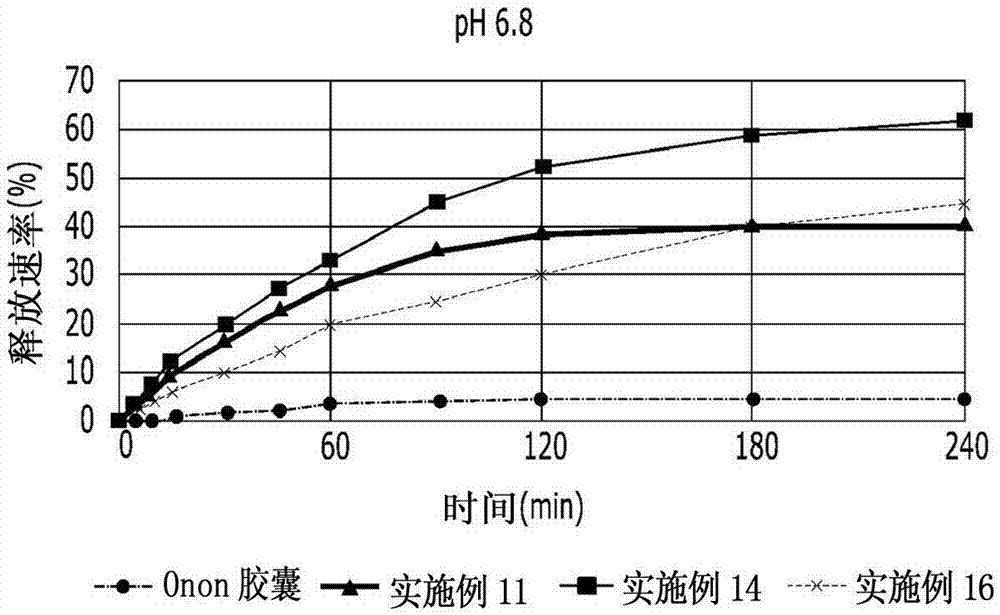
[0068] A tablet and two Onon capsules (pranlukast 112.5 mg / capsule, single dose of 2 capsules) were prepared according to the method 2 dissolution test of Korean Pharmacopoeia (Korean Pharmacopoeia) for each of Examples 11, 14 and 16 , Donga-ST Co., Ltd.) for a comparative dissolution test.
[0069] 900 mL of the pH 6.8 solution was put into dissolution vessels respectively, and stirred at a rotation speed of 50 rpm while maintaining a temperature of 37±0.5° C. to measure the release rate. At each point, approximately 5 mL of the solution was withdrawn, filtered through a 0.45 μm filter and analyzed by HPLC. The result is as figure 1 shown.
[0070] Depend on figure 1 It can be seen that the release rate of the tablet prepared from the wet granulation according to the invention is significantly increased compared to Onon capsules as a commercial formulation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com