Sustained release tablet for curing gastritis and preparation method thereof
A technology of slow-release tablets and gastritis, applied in the field of slow-release tablets for the treatment of gastritis and its preparation, to achieve good curative effect, reduce adverse reactions, and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of the sustained-release tablet for the treatment of gastritis, comprises the following steps:
[0030] S1: Weigh the components according to parts by weight: 20kg of open arrow, 10kg of double-faced needle, 10kg of mulixiang, 6kg of tangerine core, and 6kg of licorice;
[0031] S2: Add the weighed open arrow, double-sided needle, Jiulixiang, orange core, and licorice, add 10 times the amount of water to decoct and extract once, decoct for 3 hours, filter, combine the filtrate, concentrate, granulate, dry, Pulverize to get dry cream powder;
[0032] S3: Add 5 kg of hydroxypropyl methylcellulose, 0.8 kg of polyvinylpyrrolidone, 3 kg of microcrystalline cellulose, and 0.5 kg of magnesium stearate to the above dry cream powder, mix well, and press into tablets to obtain the product.
Embodiment 2
[0034] The preparation method of the sustained-release tablet for the treatment of gastritis, comprises the following steps:
[0035] S1: Weigh the components according to parts by weight: 50kg of open arrow, 30kg of double-faced needle, 20kg of mulixiang, 15kg of tangerine core, and 15kg of licorice;
[0036] S2: Add the weighed Kaijianjian, LMZ, Jiulixiang, Tangerine Pit, and Licorice, add 6 times the amount of water to decoct and extract 3 times, decoct for 1 hour each time, filter, combine the filtrate, concentrate, and granulate, Dried and crushed to obtain dry cream powder;
[0037] S3: add 10 kg of hydroxypropyl methylcellulose, 1.2 kg of polyvinylpyrrolidone, 8 kg of microcrystalline cellulose, and 0.8 kg of magnesium stearate to the above dry cream powder, mix well, and press into tablets to obtain the product.
Embodiment 3
[0039] The preparation method of the sustained-release tablet for the treatment of gastritis, comprises the following steps:
[0040] S1: Weigh the components according to parts by weight: 35kg of open arrow, 20kg of double-faced needle, 15kg of mulberry, 12kg of orange core, and 9kg of licorice;
[0041] S2: Add the weighed Kaijianjian, Liangmianzhen, Jiulixiang, Tangerine Pit, and Licorice, add 8 times the amount of water to decoct and extract twice, decoct for 2 hours each time, filter, combine the filtrate, concentrate, and granulate, Dried and crushed to obtain dry cream powder;
[0042] S3: Add 8 kg of hydroxypropyl methylcellulose, 1 kg of polyvinylpyrrolidone, 5 kg of microcrystalline cellulose, and 0.6 kg of magnesium stearate to the above dry cream powder, mix well, and press into tablets to obtain the product.
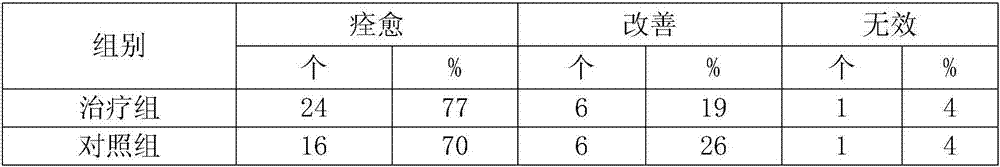
[0043] 2. Clinical application
[0044] 1. Case selection criteria
[0045] All cases were in line with the diagnostic criteria of "Endoscopic Classifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com