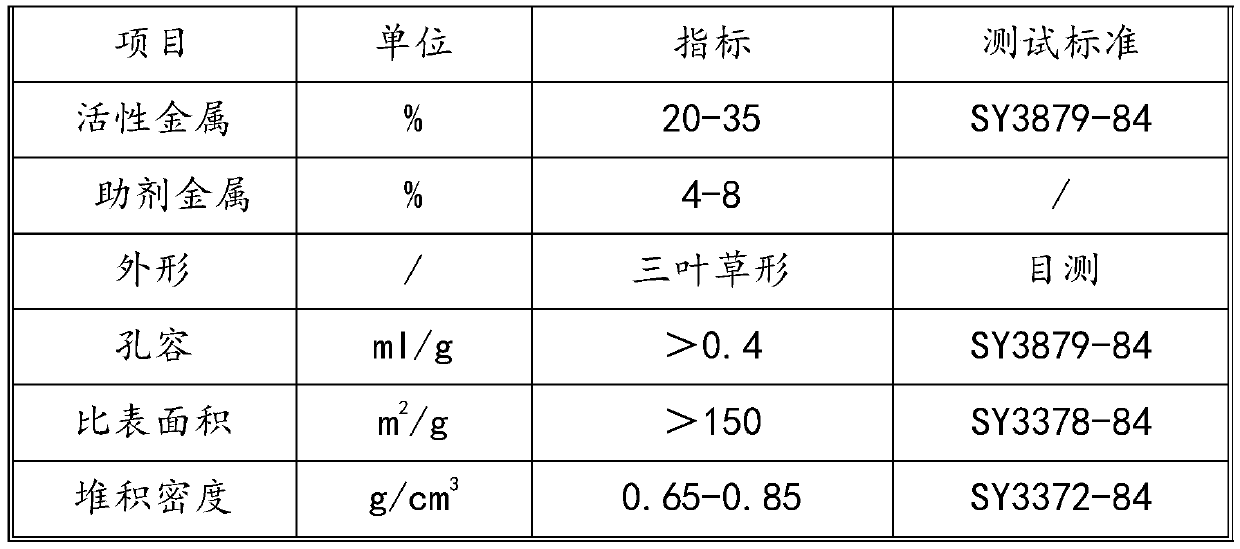
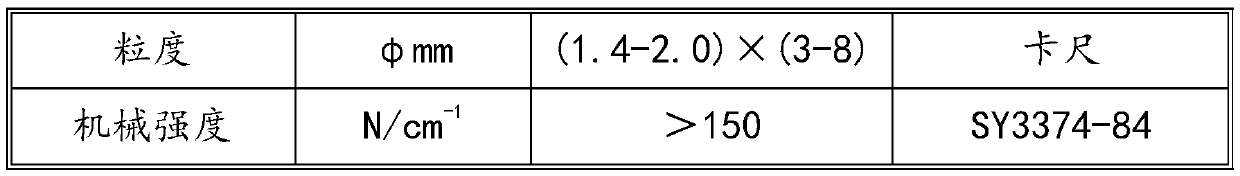
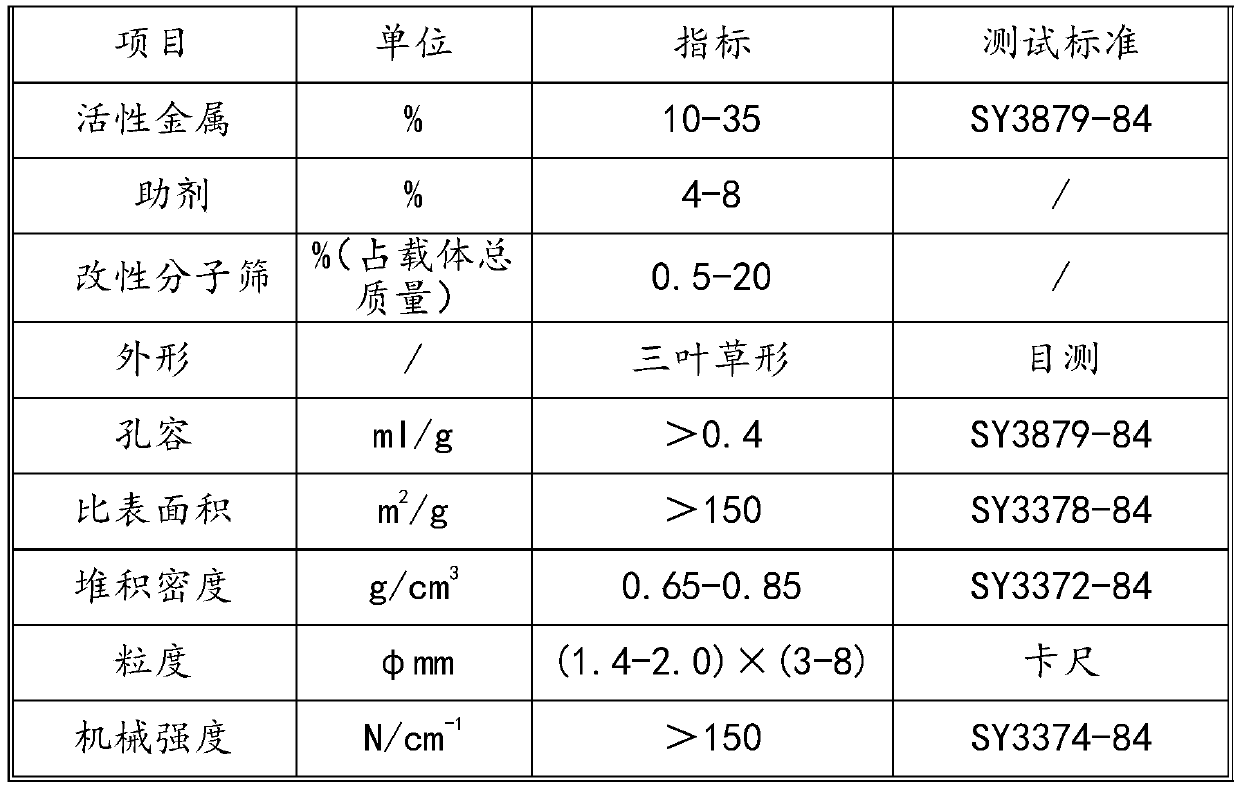
Method for preparing fuel oil by catalytic hydrogenation of shale oil
A technology for catalytic hydrogenation and shale oil, applied in the chemical industry, can solve the problems of blocking catalyst pores or catalyst beds, easily destroying the acid center of the hydrogenation catalyst, and high production costs, and reducing the discharge of sulfur-containing sewage. , the effect of reducing energy consumption and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1 raw material oil property and hydrogenation result are as shown in table 4 below:
[0057] Table 4 Example 1 Raw Oil Properties and Hydrogenation Results
[0058]
[0059]
[0060] Note: When the process parameters of the three reactors are consistent, only one value is shown.
Embodiment 2
[0062] The stock oil that embodiment 2 uses is identical with embodiment 1. The properties of raw oil and hydrogenation results are shown in Table 5 below:
[0063] Table 5 Example 2 Raw Oil Properties and Hydrogenation Results
[0064]
[0065]
[0066] It can be seen from Table 4 and Table 5 that the sulfur in shale oil hydrogenation products can be made -1 , Nitrogen content -1 .
[0067] Adopt the raw material oil identical with embodiment 1, use different reaction conditions respectively to obtain embodiment 3-5, and its result is as shown in table 6 below:
[0068] Table 6 embodiment 3-5 reaction result
[0069]
[0070]
[0071] In order to illustrate the importance of the selection of reaction conditions, the same method was used, the reaction parameters were changed, and comparative experiments 1-3 were carried out. The results are shown in Table 7 below:
[0072] Table 7 Comparative Examples 1-3 Results
[0073]
[0074]
[0075] The results of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com