Preparation method of 2-fluorine-3-chlorine-5-trifluoromethylpyridine
A technology of trifluoromethylpyridine and trichloromethylpyridine, which is applied in the field of preparation of 2-fluoro-3-chloro-5-trifluoromethylpyridine, can solve the problem of difficulty in separating isomers, difficulty in industrialized production, and side effects. There are many problems such as many products, so as to achieve the effect of simple operation, favorable for large-scale production and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
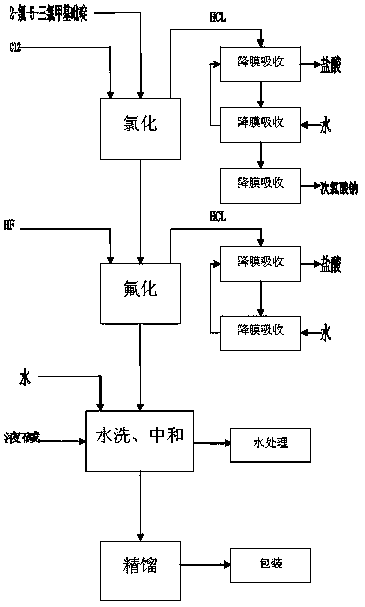
[0041] Put 2000kg of 2-chloro-5-trichloromethylpyridine into the glass-lined chlorination kettle, then add the catalyst into the reaction kettle and heat the 150kg reaction kettle with heat-conducting oil. When the temperature rises to 130°C, feed chlorine gas into the reaction kettle , Control the amount of chlorine, so that the reaction temperature is controlled at 180 ° C. The reaction time is generally 100 hours. After the reaction is completed, the temperature is lowered to 50°C, the catalyst is filtered out, and 2,3-dichloro-5-trichloromethylpyridine is metered into the fluorination tank for fluorination.
[0042] Put the chlorinated 2,3-dichloro-5-trichloromethylpyridine into the fluorination kettle (about 2300kg), put the catalyst antimony pentafluoride 200kg into the reaction fluorine, add 1700kg of anhydrous hydrogen fluoride, press 2,3 - The molar ratio of dichloro-5-trichloromethylpyridine to hydrogen fluoride is 1:10, and excess hydrogen fluoride is beneficial to ...
Embodiment 2
[0047] Put 2000kg of 2-chloro-5-trichloromethylpyridine into the glass-lined chlorination kettle, then add the catalyst into the reaction kettle and heat the 150kg reaction kettle with heat-conducting oil. When the temperature rises to 130°C, feed chlorine gas into the reaction kettle , Control the amount of chlorine, so that the reaction temperature is controlled at about 190 ° C. The reaction time is generally 70 hours. After the reaction is completed, the temperature is lowered to 50°C, the catalyst is filtered out, and 2,3-dichloro-5-trichloromethylpyridine is metered into the fluorination tank for fluorination.
[0048]Put the chlorinated 2,3-dichloro-5-trichloromethylpyridine into the fluorination kettle (about 2300kg), put the catalyst antimony pentafluoride 200kg into the reaction fluorine, add 1700kg of anhydrous hydrogen fluoride, press 2,3 - The molar ratio of dichloro-5-trichloromethylpyridine to hydrogen fluoride is 1:12, and excess hydrogen fluoride is beneficial...
Embodiment 3
[0051] Put 2000kg of 2-chloro-5-trichloromethylpyridine into the glass-lined chlorination kettle, then add the catalyst into the reaction kettle and heat the 150kg reaction kettle with heat-conducting oil. When the temperature rises to 130°C, feed chlorine gas into the reaction kettle , Control the amount of chlorine, so that the reaction temperature is controlled at about 185 ° C. The reaction time is generally 85 hours. After the reaction is completed, the temperature is lowered to 50°C, the catalyst is filtered out, and 2,3-dichloro-5-trichloromethylpyridine is metered into the fluorination tank for fluorination.
[0052] Put the chlorinated 2,3-dichloro-5-trichloromethylpyridine into the fluorination kettle (about 2300kg), put the catalyst antimony pentafluoride 200kg into the reaction fluorine, add 1700kg of anhydrous hydrogen fluoride, press 2,3 - The molar ratio of dichloro-5-trichloromethylpyridine to hydrogen fluoride is 1:11, and excess hydrogen fluoride is beneficia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com