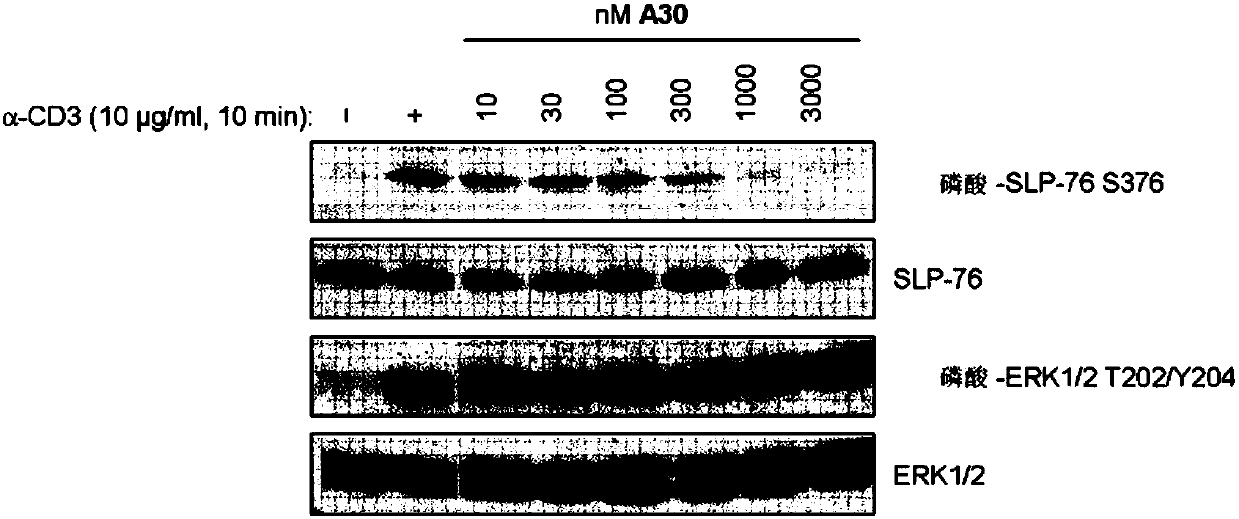
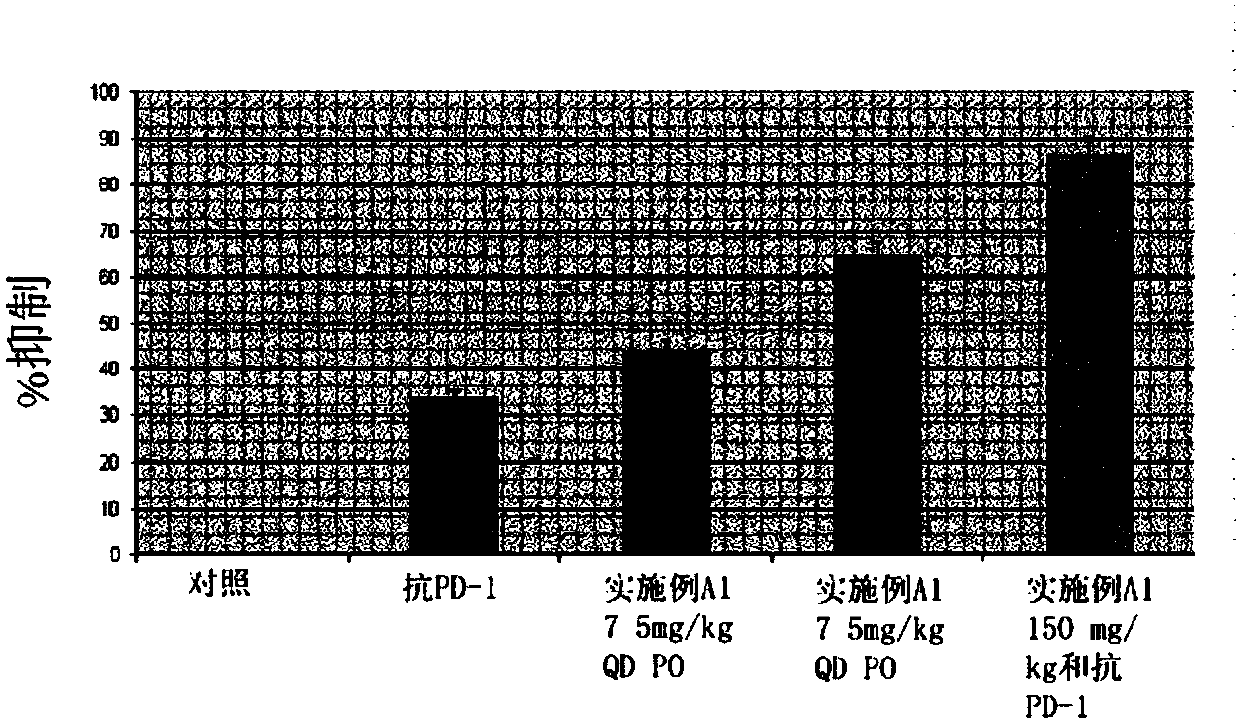
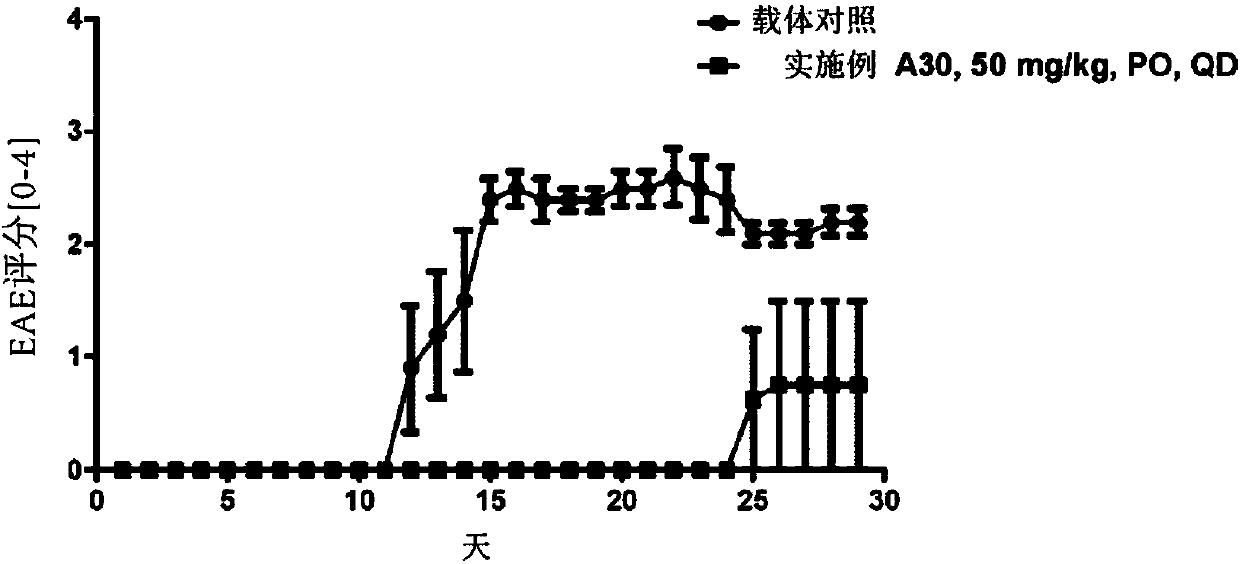
HPK1 inhibitors and methods of using same
A CH2, -NH2 technology, applied in organic chemical methods, chemical instruments and methods, pharmaceutical formulations, etc., can solve problems such as cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0110] Example A: Synthesis
[0111] general method
[0112] Commercially available starting materials, reagents, and solvents were used as received. Generally, anhydrous reactions are performed under an inert atmosphere such as nitrogen or argon. Rxn CX refers to commercially available cation exchange resins available from Waters.
[0113]Microwave reactions were performed using a Biotage Initiator microwave reactor. Reaction progress was generally monitored by LCMS (Bruker Exquire 4000 or Waters Acquity UPLC system). Flash column chromatography purification of intermediates or final products was carried out using Biotage Isolera with KP-SIL or HP-SIL silica cartridges, or KP-NH basic modified silica and corresponding samplers (samplets) . Reverse-phase HPLC purification was carried out on a Varian PrepStar SD-1 HPLC system with a Varian Monochrom 10μC-18 reverse-phase column, using 10% MeOH / 0.05% TFA-H 2 O to H 2 A gradient of 90% MeOH / 0.05% TFA in O was performed ov...
Embodiment B
[0308] Example B: HPK1 Inhibition Assay
[0309] Active HPK1 (MAP4K1 ) was purchased from Invitrogen (cat#PV6355) as an N-terminal GST fusion of human HPK1 (aa 1-346). HPK1 activity was measured using an indirect ELISA detection system. GST-HPK1 (0.6nM) in the presence of 12μM ATP (Sigma cat#A7699), 5mM MOPS (pH 7.2), 2.5mM β-glycerol-phosphate, 5mM MgCl 2 , 0.4 mM EDTA, 1 mM EGTA, 0.05 mM DTT in 96-well microtiter plates pre-coated with 0.5 μg / well bovine myelin basic protein (MBP) (Millipore, cat#13-110). The reaction was carried out for 30 min, and then the plate was washed 5 times with washing buffer (phosphate-buffered saline supplemented with 0.2% Tween 20), and mixed with 1:1 of anti-phospho-threonine rabbit polyclonal antibody (Cell Signalingcat #9381). 3000 dilutions were incubated together for 30min. Plates were washed 5 times with wash buffer, incubated for 30 min in the presence of goat anti-rabbit horseradish peroxidase conjugate (BioRad cat#1721019, 1:3000 con...
Embodiment C
[0311] Example C: FLT3 Inhibition Assay
[0312] FLT3 and LCK compound inhibition was determined using a FRET-based Z'-LYTE Kinase Assay Kit with Tyrosine 2-peptide as substrate (Invitrogen cat #PV3191). The FLT3 kinase assay was performed at a concentration of 940 μM ATP and 1 nM FLT3 (Invitrogen cat #PV3182) following the manufacturer's suggested instructions, and 180 μM ATP and 25 nM LCK (Invitrogen cat #P3043) was used for the LCK kinase reaction. % inhibition values are determined according to the manufacturer's guidelines, and the IC 50Values were obtained using a nonlinear 4-point logistic curve fit (XLfit4, IDBS).
[0313] In Table 1 below, the ICs of exemplary compounds are given 50 range of values. IC 50 Ranges denote values less than or equal to 0.05 μM; values greater than 0.05 μM and less than or equal to 0.5 μM; and values greater than 0.5 μM by “A”, “B” and “C”, respectively.
[0314] Table 1: Inhibition data of HPK1, Lck and Flt3
[0315]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com