Method for selectively preparing alpha-acyloxylation or beta-acyloxylation product of enamine ketone compound
A technology of acyloxylation and enaminone, which is applied in the field of organic synthesis methodology, can solve problems such as failure to pass, and achieve the effects of mild reaction conditions, high yield, and simple process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The first part: selective preparation of α-acyloxylation products of enaminone compounds;
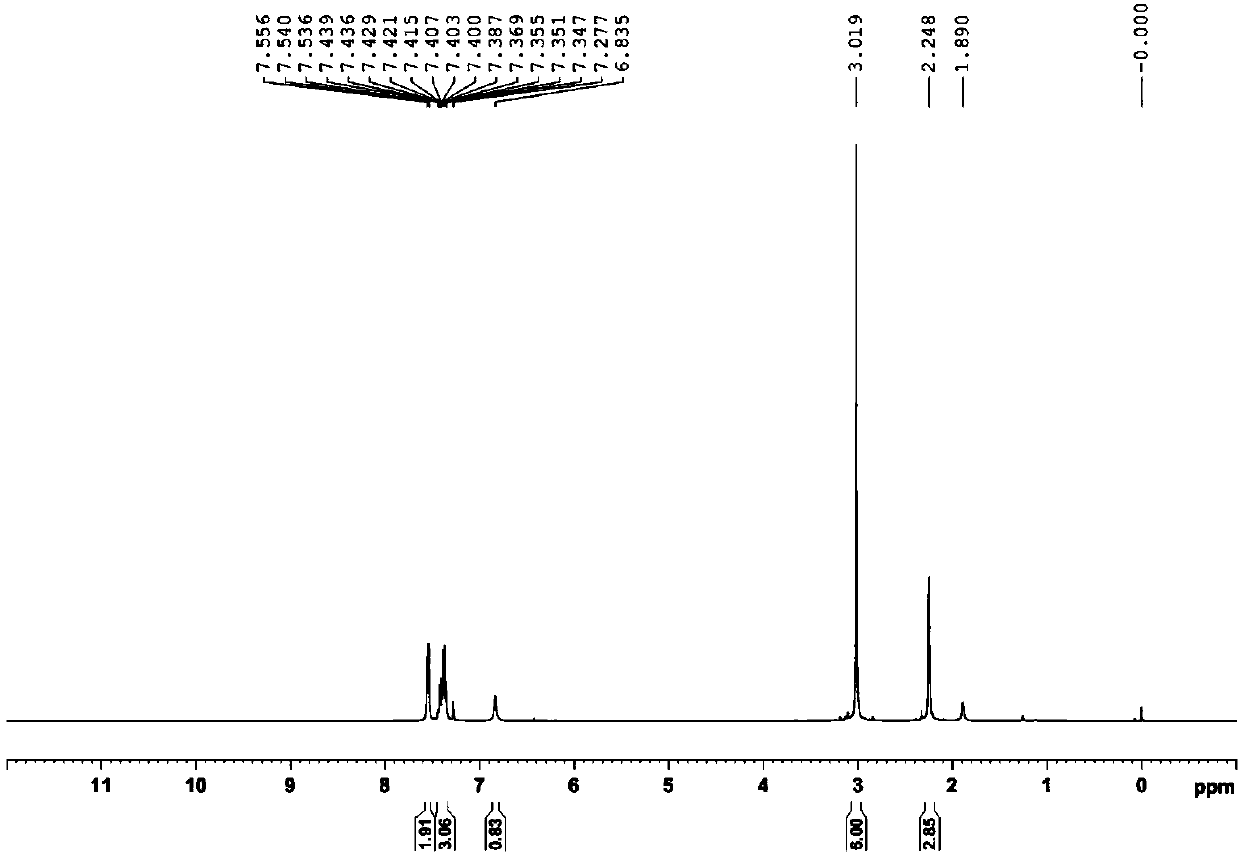
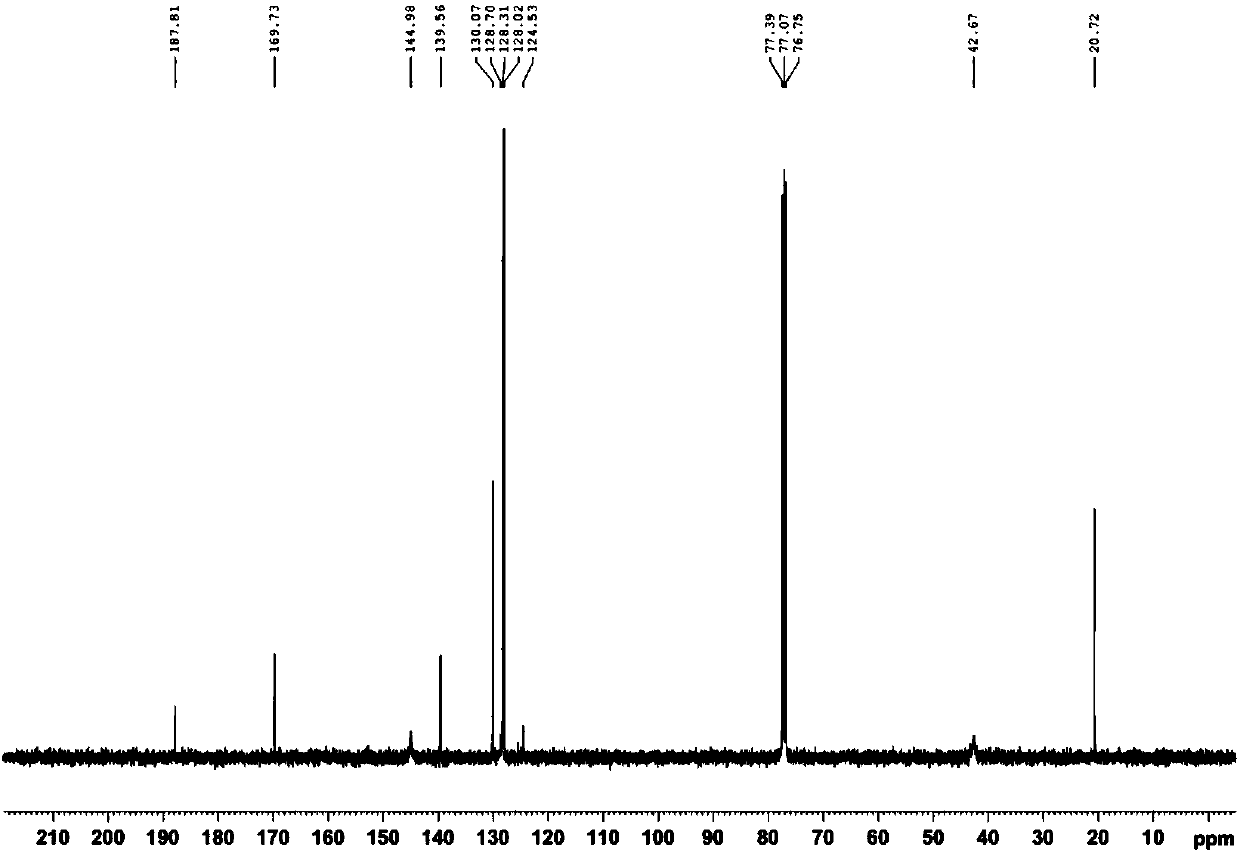
[0034] Add (E)-1-phenyl-3-dimethylamino-2-propenone and PIDA in the ratio of equivalent ratio 1.0:1.3 (weigh 0.20mmol and 0.26mmol respectively) into the round bottom flask, then add the reaction The solvent trifluoroethanol (TFE) (2.0mL) is stirred in air at room temperature for 0.5 to 3 hours to selectively obtain compound (Z)-1-phenyl-2-acetoxy-3-dimethylamine Base-2-propenone, the yield is 83%; 1 H NMR spectrum see figure 1 , 13 C NMR spectrum see figure 2 .
[0035] The second part: selective preparation of β-acyloxylation products of enaminone compounds;
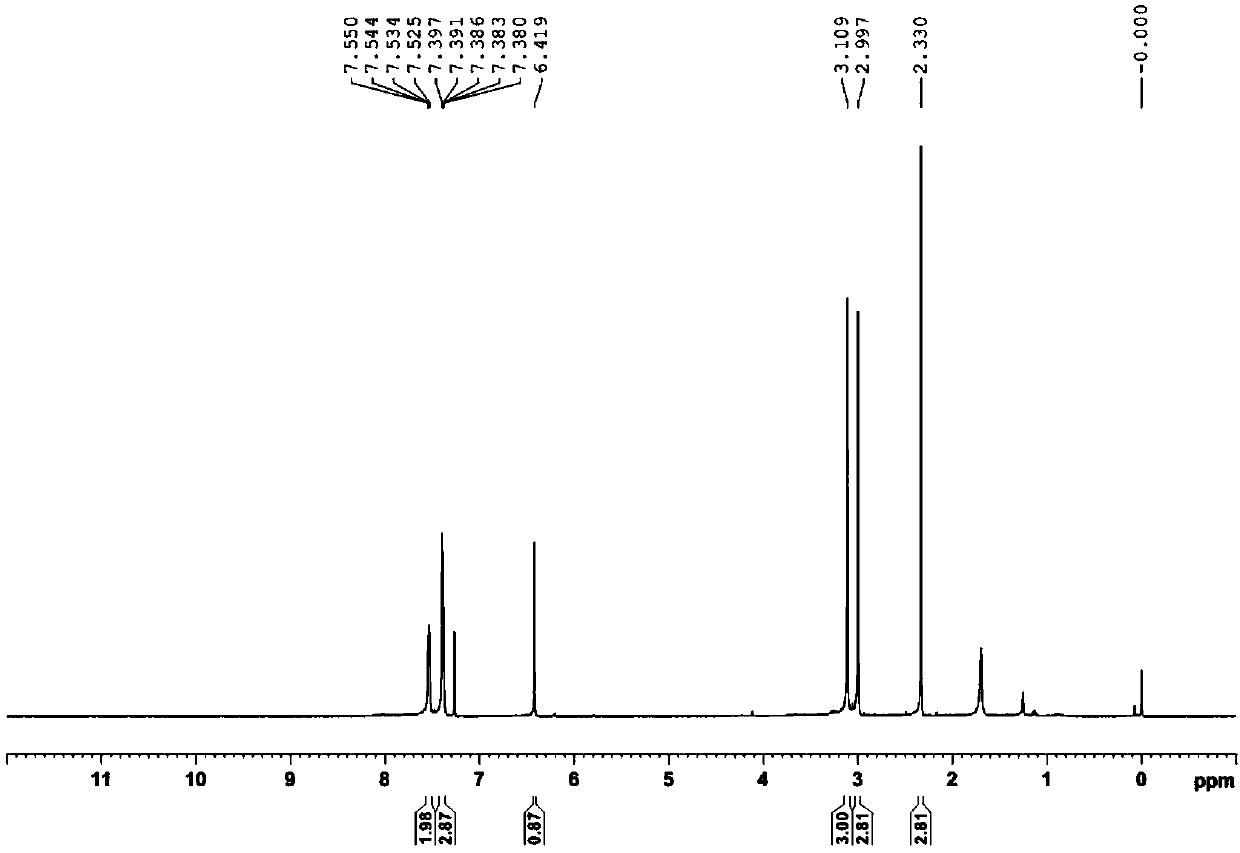
[0036] (E)-1-phenyl-3-dimethylamino-2-propenone and PIDA were added to the round bottom flask according to the ratio of equivalent ratio 1.0:2.0 (weighed 0.20mmol and 0.40mmol respectively), and then added 2.0 Equivalent water (0.40mmol) and reaction solvent acetonitrile (2.0mL), stirred in air at room temperature ...
Embodiment 2
[0038] The first part: selective preparation of α-acyloxylation products of enaminone compounds;
[0039](E)-1-phenyl-3-(1-tetrahydropyrrolyl)-2-propenone and PIDA were added to the round bottom flask according to the ratio of equivalent ratio 1.0:1.3 (weighed 0.20mmol and 0.26mmol respectively) , then add the reaction solvent trifluoroethanol (TFE) (2.0mL) and stir in the air at room temperature for 0.5 to 3 hours, the compound (Z)-1-phenyl can be selectively obtained in a higher yield of 71%. -2-Acetoxy-3-(1-tetrahydropyrrolyl)-2-propenone, the yield is 71%;
[0040] The second part: selective preparation of β-acyloxylation products of enaminone compounds;
[0041] Add (E)-1-phenyl-3-(1-tetrahydropyrrolyl)-2-propenone and PIDA in the ratio of 1.0:2.0 (weigh 0.20mmol and 0.40mmol respectively) into the round bottom flask , then add 2.0 equivalents of water (0.40mmol) and reaction solvent acetonitrile (2.0mL), and stir in air at room temperature for 3 to 6 hours to selective...
Embodiment 3
[0043] The first part: selective preparation of α-acyloxylation products of enaminone compounds;
[0044] Add (E)-1-(4-nitrophenyl)-3-dimethylamino-2-propenone and PIDA to the round bottom at the ratio of equivalent ratio 1.0:1.3 (weigh 0.20mmol and 0.26mmol respectively) In the flask, add the reaction solvent trifluoroethanol (TFE) (2.0mL) and stir in the air at room temperature for 0.5 to 3 hours to selectively obtain the compound (Z)-1-(4-nitrophenyl) -2-acetoxy-3-dimethylamino-2-propenone, the yield is 86%;
[0045] The second part: selective preparation of β-acyloxylation products of enaminone compounds;
[0046] Add (E)-1-(4-nitrophenyl)-3-dimethylamino-2-propenone and PIDA to the round bottom at an equivalent ratio of 1.0:2.0 (weigh 0.20mmol and 0.40mmol respectively) In the flask, add 2.0 equivalents of water (0.40mmol) and reaction solvent acetonitrile (2.0mL), and stir in air at room temperature for 3 to 6 hours to selectively obtain compound (Z)-N,N-di Methyl-3-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com