Artificial medicine carrying coaxial regenerated intravascular stent and compound process preparation method thereof
A vascular stent and drug-carrying technology, applied in blood vessels, stents, pharmaceutical formulations, etc., can solve the problems of reducing the infection rate of artificial blood vessels, shortening the preparation time of stents, etc., so as to improve mechanical and biological properties, reduce infection risks, and increase tissue phase. capacitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
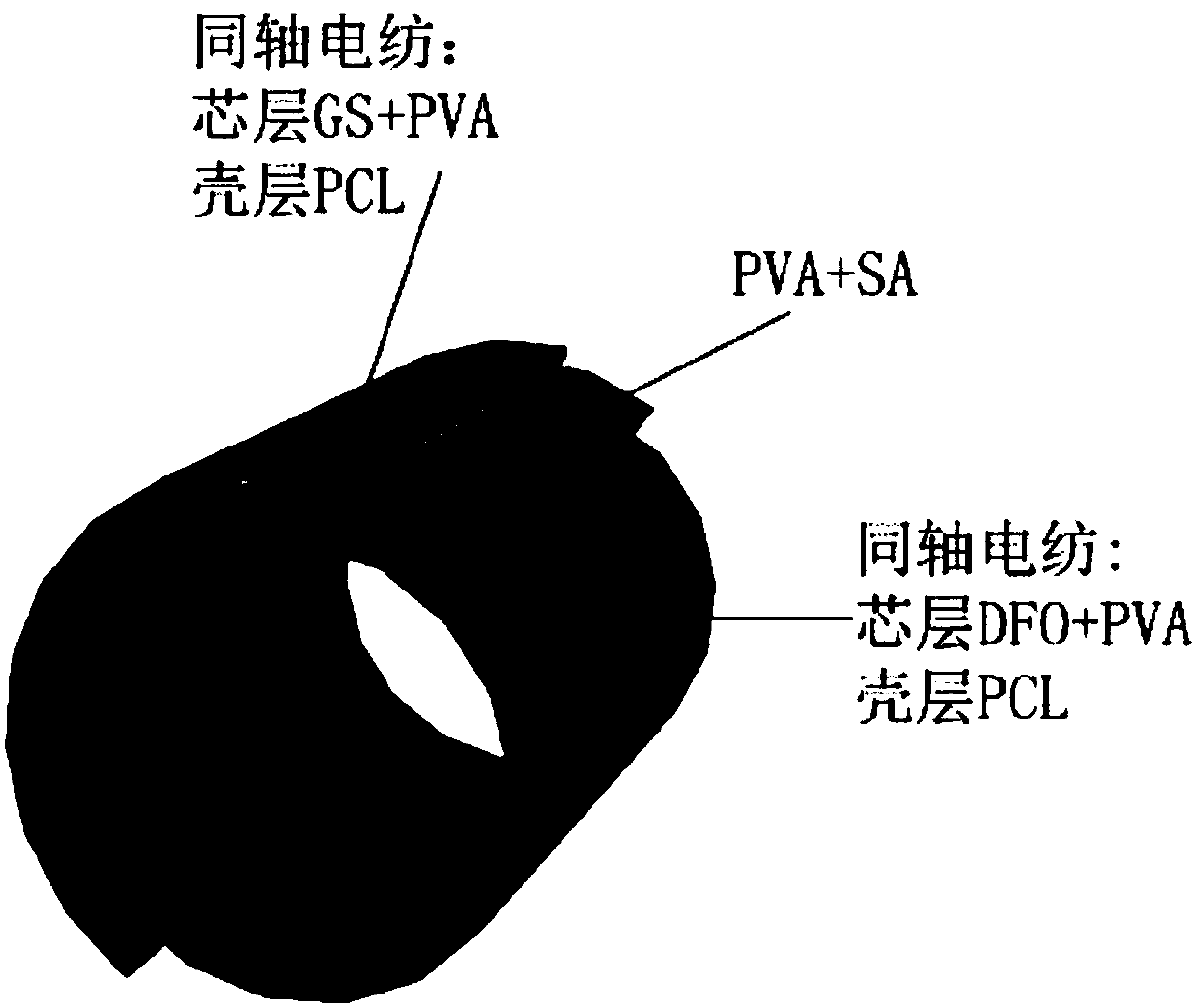
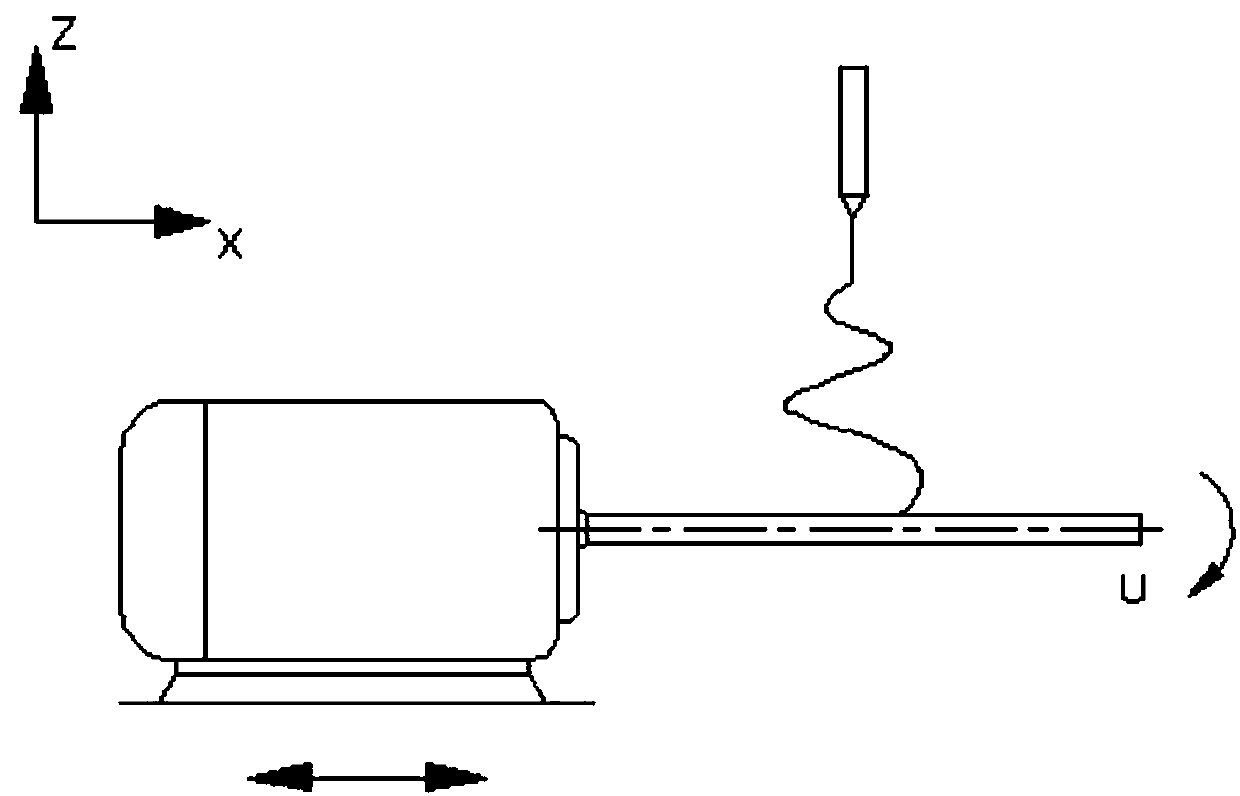
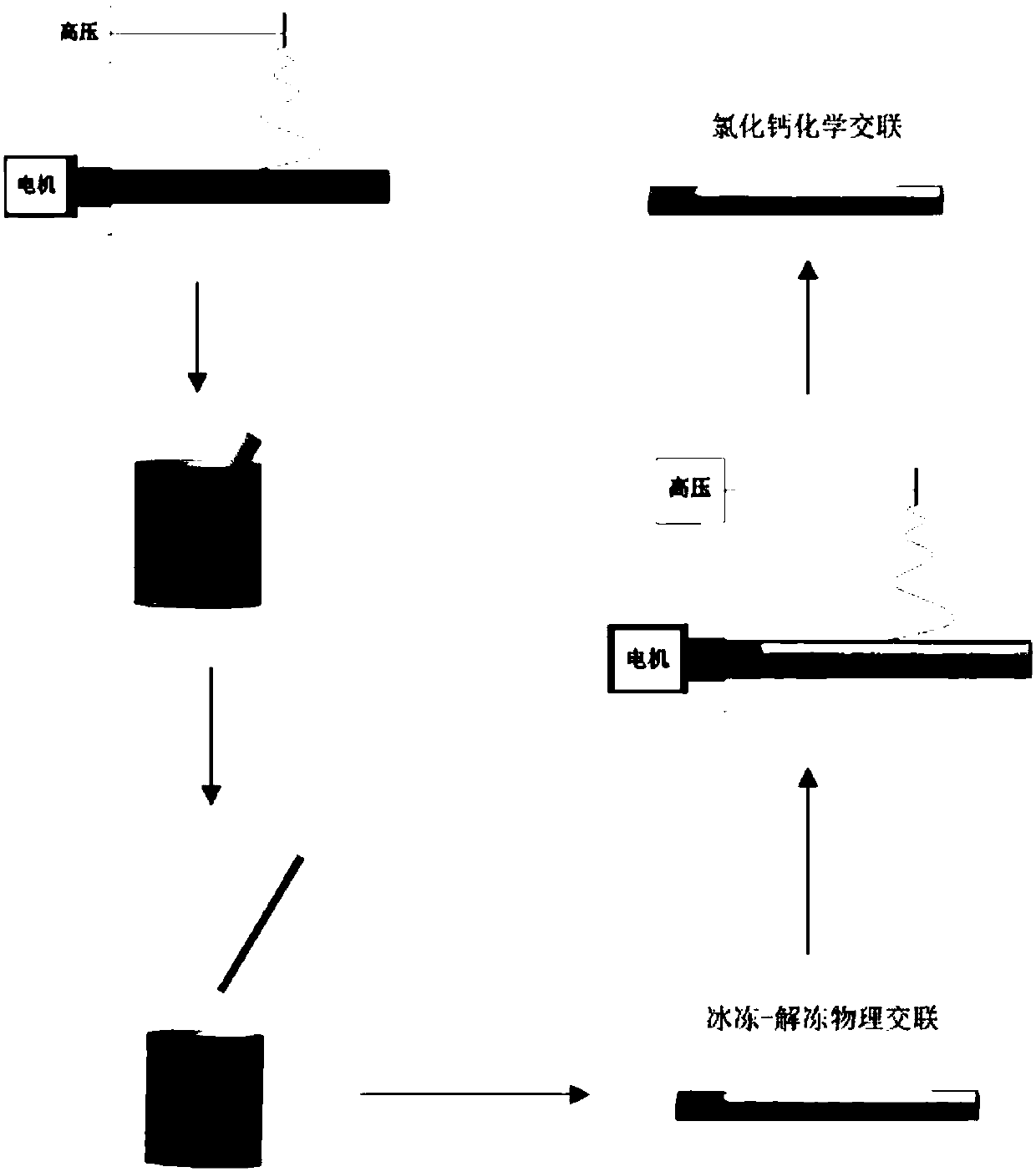
[0037] In this example, see Figure 1~3 , a method for preparing a drug-loaded coaxial regenerative vascular stent by a composite process, comprising the steps of:
[0038] a. PVA is dissolved in deionized water, heated in a water bath on a magnetic stirrer, and stirred until the PVA is completely dissolved, and the obtained mass percent concentration is 5wt.% PVA solution 35g;
[0039] b. Dissolve 20 mg of deferoxamine DFO in 10 g of the PVA solution prepared in step a to prepare the DFO inner layer coaxial electrospinning core layer solution;
[0040] c. Use a mixture of N,N-dimethylformamide DMF and dichloromethane DCM with a volume ratio of 1:1 as a solvent, dissolve 2g polycaprolactone PCL in 20ml of the above solvent, and prepare a PCL coaxial electrode Spin shell layer solution, divide into two equally again;
[0041] d. Sodium alginate SA is dissolved in deionized water to prepare 10 g of SA solution with a mass fraction of 3wt.%, and then the PVA solution and SA solut...
Embodiment 2
[0049] This embodiment is basically the same as Embodiment 1, especially in that:
[0050] In this embodiment, a method for preparing a drug-loaded coaxial regenerated vascular stent by a composite process includes the following steps:
[0051] a. PVA is dissolved in deionized water, heated in a water bath on a magnetic stirrer, and stirred until the PVA is completely dissolved, and the obtained mass percent concentration is 3wt.% PVA solution 35g;
[0052] b. Dissolve 20mg of deferoxamine DFO in the PVA solution prepared in the step a, and prepare a DFO inner layer coaxial electrospinning core layer solution with a mass percentage of 50wt.%.
[0053] c. Use a mixture of N,N-dimethylformamide DMF and dichloromethane DCM with a volume ratio of 1:1 as a solvent, dissolve 0.2g polycaprolactone PCL in 20ml of the above solvent, and prepare a PCL coaxial The electrospinning shell solution was divided into two equally;
[0054] d. Sodium alginate SA is dissolved in deionized water...
Embodiment 3
[0062] This embodiment is basically the same as the previous embodiment, and the special features are:
[0063] In this embodiment, a method for preparing a drug-loaded coaxial regenerated vascular stent by a composite process includes the following steps:
[0064] a. PVA is dissolved in deionized water, heated in a water bath on a magnetic stirrer, and stirred until the PVA is completely dissolved, and the obtained mass percent concentration is 8wt.% PVA solution 35g;
[0065] b. Dissolve 20mg deferoxamine DFO in the PVA solution prepared in the step a, and prepare a DFO inner layer coaxial electrospinning core layer solution with a mass percentage of 80wt.%.
[0066] c. Use a mixture of N,N-dimethylformamide DMF and dichloromethane DCM with a volume ratio of 1:1 as a solvent, dissolve 2g polycaprolactone PCL in 20ml of the above solvent, and prepare a PCL coaxial electrode Spin shell layer solution, divide into two equally again;
[0067] d. Sodium alginate SA is dissolved...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com