Oncolytic adenoviruses with mutations in immunodominant adenovirus epitopes and their use in cancer treatment
An oncolytic adenovirus and adenovirus technology, applied in the field of oncolytic adenovirus, can solve problems such as accelerated virus rapid release, accelerated virus transmission, and low total virus yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0176] It should be understood that the examples and implementations described herein are for illustrative purposes only, and various modifications or changes may be made by those skilled in the art, and shall be included within the spirit and scope of the present application.
[0177] Examples 1-4 demonstrate the immunological shift of the immune response from anti-adenovirus to anti-tumor by deletion of immunodominant T cell epitopes of adenovirus. ICO15K-TD-gp100-tyr (which is an HAd5 oncolytic adenovirus containing mutations in the three immunodominant T cell epitopes of the hexon and the human tumor epitope gp100-tyr inserted in the hexon) was used , demonstrated that mice treated with ICO15K-TD-gp100-tyr had attenuated overall resistance to adenovirus compared to mice treated with ICO15K-gp100-tyr, a control virus lacking the three hexon mutations immune response, while showing a higher immune response to the tyr tumor epitope.
Embodiment 5
[0178] Example 5 shows that ICO15K-TD-gp100-tyr induces more potent antitumor activity than ICO15K-gp100-tyr in murine tumor-bearing animals. Specifically, animals injected with ICO15K-TD-gp100-tyr were less likely to form tumors than animals treated with ICO15K-gp100-tyr.
[0179] Examples 6-8 show the effect of immunotransfer on antitumor activity in the absence of tumor epitopes. Specifically, the quadruple-deleted (QD) ICOVIR15K adenovirus (ICOVIR15K-QD) induced more potent antitumor activity than ICO15K in murine tumor-bearing animals, where the quadruple-deleted (QD ) ICOVIR15K adenovirus (ICOVIR15K-QD) lacks human tumor epitopes and introduces additional hexon epitope mutations.
Embodiment 1
[0181] Generation of oncolytic adenovirus ICOVIR15K-TD and its derivative ICO15K-TD-gp100-tyr with triple deletion (TD) using restricted-library method
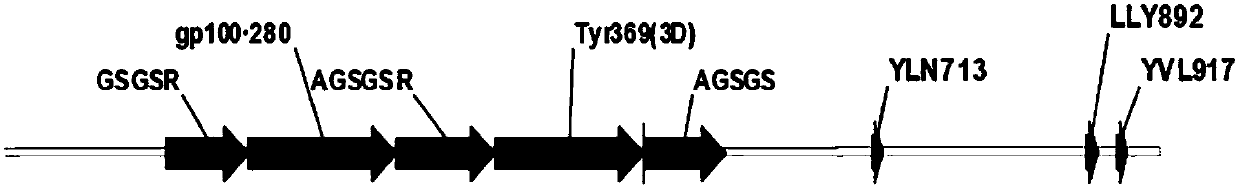
[0182]The literature was searched for immunodominant epitopes restricted by human lymphocyte antigen-A2.1 (HLA-A2.1) of adenovirus 5 through PubMed. HLA-A2.1 was chosen because it is the major histocompatibility complex (MHC) class I ( Gonzalez‐Galarza, F.F., et al., Nucleic Acids Res. 2011 Jan; 39 (Database issue): D913-9). Each epitope has two major anchor sites for HLA-A2.1 at positions 2 and 9 of the peptide, and two minor anchor sites at positions 1 and 3. The hexon is considered to be the most immunogenic protein of human adenovirus 5, and the following three epitopes are based on different studies (Leen, A.M., et al., Blood. 2004 Oct 15; 104(8): 2432 -40; Leen, A.M., et al., J Virol.2008Jan; 82(1):546-54; Olive, M., et al., Hum Gene Ther.2002Jul 1; 13(10):1167-78; Tang , J., et al., Virology.2006 Jul 5; 350(2):312-2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com