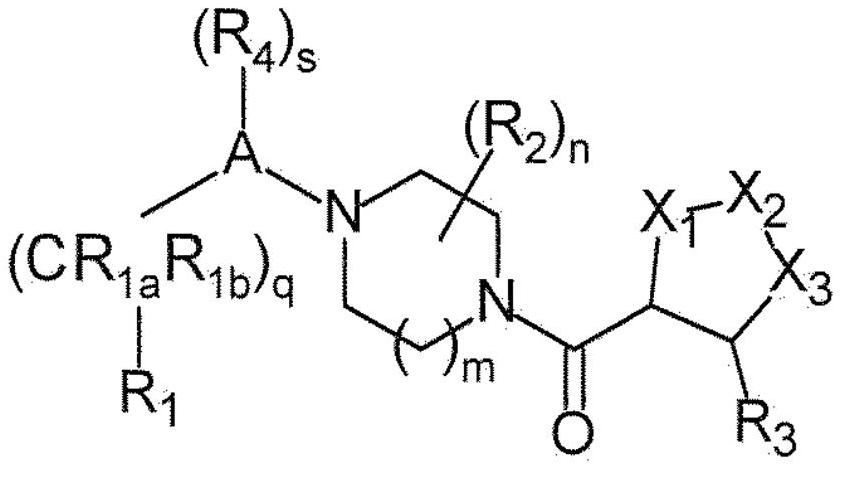
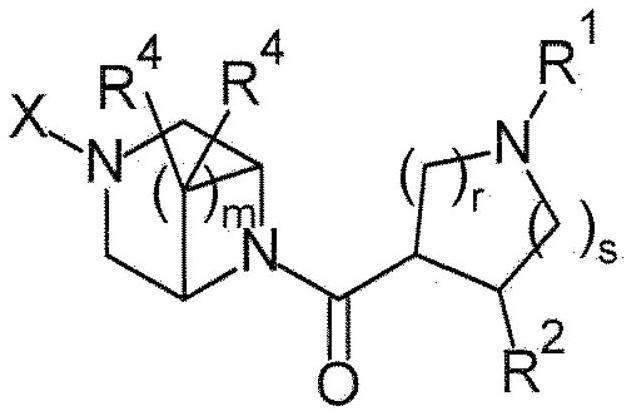
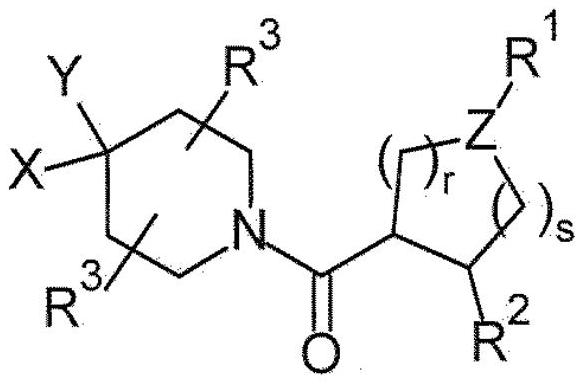
Piperazine derivatives
A technology of compound and alkyl, applied in the field of piperazine derivatives or their salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0454] Hereinafter, the production method of the compound of formula (I) is demonstrated in more detail based on an Example. It should be noted that the present invention is not limited to the compounds described in the following examples. In addition, the production method of the raw material compound is shown in the production example. In addition, the production method of the compound of formula (I) is not limited to the production method of the specific examples shown below, the compound of formula (I) can also be obtained by a combination of these production methods, or it will be obvious to those skilled in the art method to manufacture.
[0455] In addition, the following abbreviations may be used in the tables mentioned later.
[0456] PEx: production example number, Ex: example number, PSyn: production method of the production example compound (the number in the PSyn column indicates that the compound is produced by the same method as the compound having its number as...
manufacture example 1
[0464] Under nitrogen atmosphere, borane-N,N-diethylaniline complex (46.2g), (S)-5,5-diphenyl-2-methyl-3,4-propanol-1 ,3,2- A mixture of oxazaboridine (1 M in toluene, 5 mL) and tert-butyl methyl ether (250 mL) was heated to 35°C. Next, a solution of 2-chloro-1-(4-chloro-2-fluorophenyl)ethanone (51 g) in tert-butyl methyl ether (300 mL) was added dropwise at 40° C. over 2 hours. After completion of the dropwise addition, the mixture was stirred overnight while cooling to room temperature. The reaction mixture was ice-cooled, and methanol (150 mL) was added dropwise. Then, a mixture of concentrated hydrochloric acid (80 mL) and water (220 mL) was added dropwise, and stirred for 1 hour under ice-cooling. After liquid separation of the reaction mixture, the aqueous layer was extracted with tert-butyl methyl ether. The organic layers were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate. The insoluble matter was filtered off, and the filtrate...
manufacture example 2
[0466] A mixture of (1S)-2-chloro-1-(4-chloro-2-fluorophenyl)ethanol (8g) and methanol (4mL) was ice-cold, and tetrahydro-2H-pyran-4-amine (20mL) was added and sodium hydroxide (1.7 g). The reaction mixture was stirred overnight at 60 °C.
[0467] After cooling the reaction mixture to room temperature, it was poured into water (320 mL), and stirred at room temperature for 1 hour. The generated solid was collected by filtration, and the obtained solid was dried at 50° C. under reduced pressure. The obtained solid was added to a mixed solution of hexane (160 mL) and diisopropyl ether (16 mL), stirred at 70° C. for 4 hours, and stirred overnight while cooling to room temperature. The solid was collected by filtration and dried under reduced pressure at 50°C, thus obtaining (1S)-1-(4-chloro-2-fluorophenyl)-2-(tetrahydro-2H-pyran) as a solid -4-ylamino)ethanol (7.90 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com