Application of Bawei Swertia Composition in Preparation of Drugs for Treating Atrophic Gastritis
A technology of atrophic gastritis and swede, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
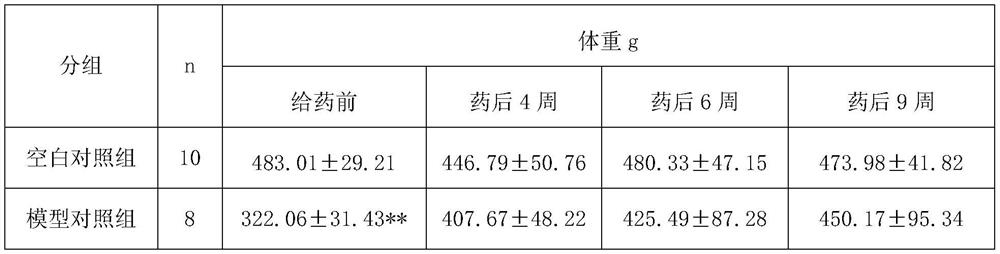
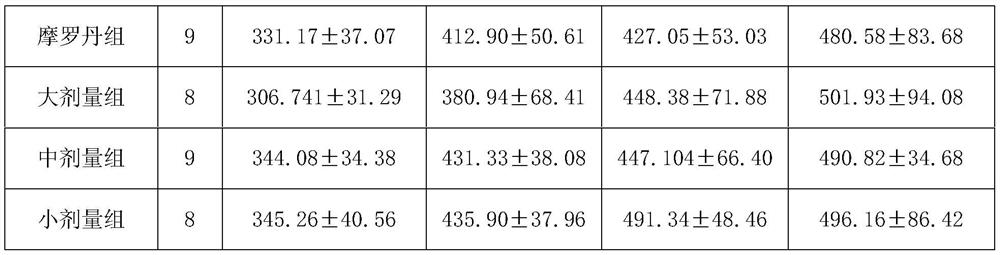
[0015] Experimental Example 1: Animal experiment on treating atrophic gastritis with Bawei Zhangyacai Pill
[0016] 1 Experiment purpose:
[0017] To observe the therapeutic effect of Bawei Zhangyacai Pill on MNNG-induced chronic atrophic gastritis (CAG) in rats.
[0018] 2 drugs and reagents
[0019] 2.1 Experimental animals
[0020] SPF grade Wistar rats, half male and half male, license number: SCXK (Lu) 20110002, provided by Shandong Lukang Pharmaceutical Co., Ltd.
[0021] 2.2 Experimental drugs
[0022] Test drug: Bawei Swertia Pills, specification 0.24g / pill, batch number: 20121024, provided by Jinhe Tibetan Medicine Co., Ltd. The daily dosage for adults is 2.4g, and the standard adult body weight is calculated as 60kg, so the clinical daily dosage for adults is 0.04g / kg. Prepare a 1g / ml suspension with distilled water just before use.
[0023] Positive control drug: Moluodan, Handan Moluodan Pharmaceutical Co., Ltd., batch number: 110417. The daily dosage of Mol...
experiment example 2
[0103] Experimental Example 2: Clinical Observation of Bawei Zhangyacai Pills in Treating Atrophic Gastritis
[0104] 1 Materials and methods
[0105] 1.1 Inclusion and exclusion criteria
[0106] 1.1.1 Inclusion criteria:
[0107] ①Meet the diagnostic criteria for chronic atrophic gastritis;
[0108] ② Chronic atrophic gastritis was diagnosed by gastroscopy and pathological examination within 1 month before inclusion in the trial;
[0109] ③Age between 19-70 years old, regardless of gender. ④Voluntary acceptance of the drug treatment, informed consent.
[0110] 1.1.2 Exclusion criteria:
[0111] ① Those who are under 19 years old or over 70 years old, pregnant or breastfeeding women, allergic constitution and allergic to this drug;
[0112] ② Combined with peptic ulcer, severe dysplasia or suspected malignant transformation;
[0113] ③ Patients with serious primary diseases such as heart, brain, liver, kidney and hematopoietic system.
[0114] 1.2 General information ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com