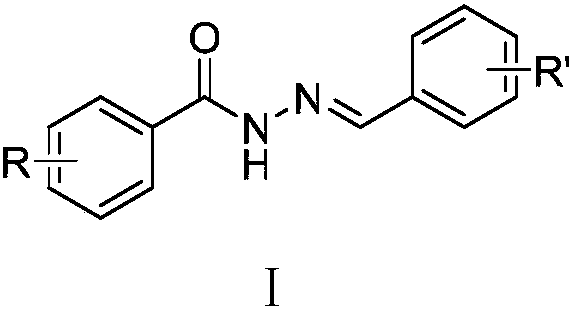
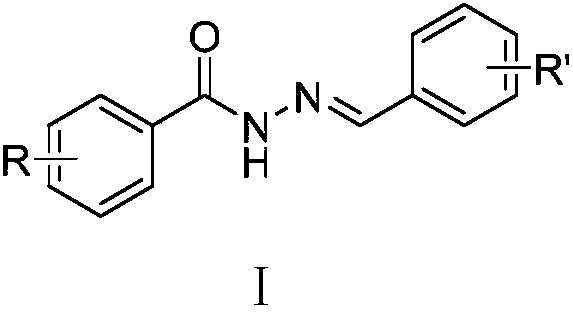
Acylhydrazone compounds with anti-tumor activity as well as preparation method and application of acylhydrazone compounds
A technology of anti-tumor drugs and compounds, applied in the preparation of hydrazones, anti-tumor drugs, organic active ingredients, etc., can solve long-term problems and achieve good inhibitory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
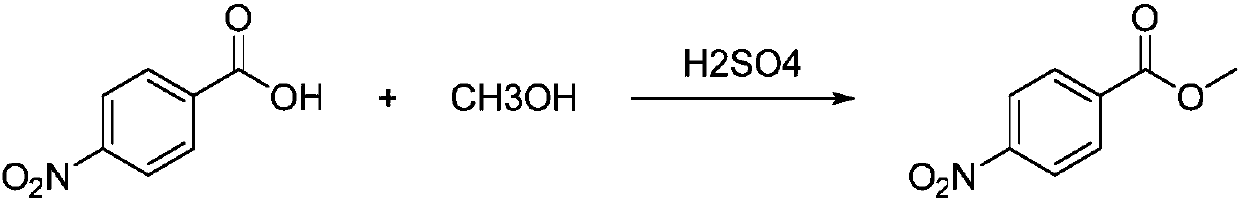
[0023] The synthesis of embodiment 1 4-methyl nitrobenzoate
[0024] Take a 25ml single-necked bottle, add 5ml of methanol and 0.4g of M1 in the following formula in sequence, stir, slowly add 1ml of concentrated sulfuric acid dropwise, and reflux at 80°C for 3h. After the reaction was completed, the methanol was spin-dried under reduced pressure, the pH was adjusted to 9 by adding a saturated sodium carbonate solution dropwise, 5ml of dichloromethane was added, the layers were separated, the organic layer was washed 3 times with saturated sodium chloride, the aqueous layer was combined, and dichloromethane extracted two Once, the organic layers were combined, and the organic phase was dried over anhydrous sodium sulfate for 1 hour. The ratio of petroleum ether to ethyl acetate was 5:1 and separated through a silica gel column to obtain M1' with a yield of 95%.
[0025] The overall reaction formula is as follows:
[0026]
Embodiment 2
[0027] Synthesis of Example 2 (1,1'-biphenyl)-4-carboxylic acid methyl ester
[0028] Take a 25ml single-necked bottle, add 5ml of methanol and 0.5g of M2 in the following formula successively, stir, slowly add 1ml of concentrated sulfuric acid dropwise, and reflux at 80°C for 3h. After the reaction, the methanol was spin-dried under reduced pressure, the pH was adjusted to 9 by adding a saturated sodium carbonate solution dropwise, 5ml of dichloromethane was added, the layers were separated, the organic layer was washed 3 times with saturated sodium chloride, the aqueous layer was combined, and dichloromethane extracted two Once, the organic layers were combined, and the organic phase was dried over anhydrous sodium sulfate for 1 hour. The ratio of petroleum ether to ethyl acetate was 5:1 and separated through a silica gel column to obtain M2' with a yield of 96%.
[0029] The overall reaction formula is as follows:
[0030]
Embodiment 3
[0031] Example 3 Synthesis of 4-amino-3-methoxybenzoic acid methyl ester
[0032] Take a 25ml single-necked bottle, add 5ml of methanol and 0.5g of 4-amino-3-methoxybenzoic acid in sequence, stir, slowly add 1ml of concentrated sulfuric acid dropwise, and react under reflux at 80°C for 3h. After the reaction was monitored by TLC, methanol was spin-dried under reduced pressure, and saturated sodium carbonate solution was added dropwise to adjust the pH to 9, and 5ml of dichloromethane was added for liquid separation. Extract twice, combine the organic layers, and dry the organic phase with anhydrous sodium sulfate for 1 hour. The ratio of petroleum ether to ethyl acetate was 4:1 and separated through a silica gel column to obtain 0.52 g of off-white solid methyl 4-amino-3-methoxybenzoate with a yield of 96%.
[0033] The overall reaction formula is as follows:
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com