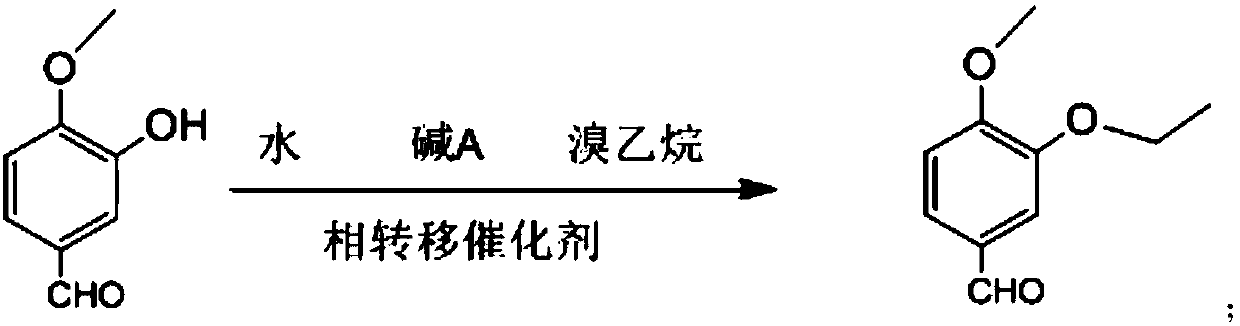
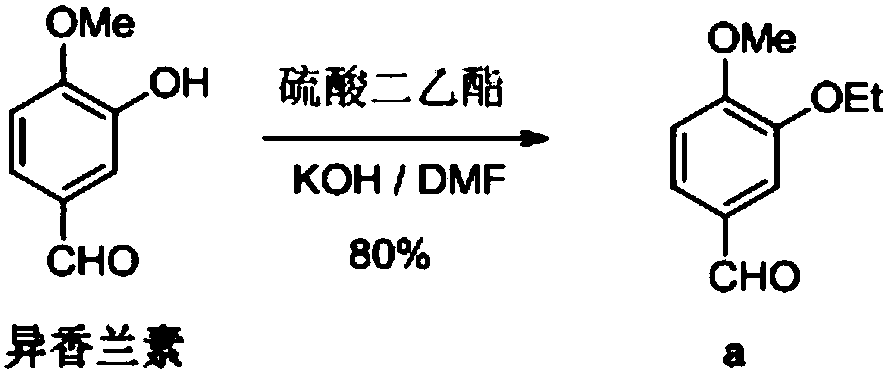
Synthesis method of 3-ethyoxyl-4-methoxybenzaldehyde
A technology of methoxybenzaldehyde and a synthetic method, which is applied in the field of synthesis of 3-ethoxy-4-methoxybenzaldehyde, can solve problems such as not being suitable for industrialized production, harming the human body or the environment, and inconvenient operation, Achieve the effects of low production cost, small human injury and easy access to reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 3L dry reaction flask, dissolve 157g of sodium hydroxide in 1500ml of water, add 500g of isovanillin, 120g of tetrabutylammonium fluoride and 537g of bromoethane, and stir the reaction at 25°C for 4h. After suction filtration, off-white solid powder 3-ethoxy-4-methoxybenzaldehyde was obtained with a purity of 99.9% and a yield of 96.1%.
Embodiment 2
[0037] In a 3L dry reaction flask, dissolve 542g of potassium carbonate in 1500ml of water, add 500g of isovanillin, 120g of tetrabutylammonium fluoride and 537g of bromoethane, stir and react at 25°C for 4h, pump Filter to obtain off-white solid powder 3-ethoxy-4-methoxybenzaldehyde with a purity of 99.8% and a yield of 95.1%.
Embodiment 3
[0039] In a 3L dry reaction flask, dissolve 157g of sodium hydroxide in 1500ml of water, add 500g of isovanillin, 104g of benzyltriethylammonium chloride and 537g of bromoethane, and stir the reaction at 25°C After 4 hours, it was filtered with suction to obtain off-white solid powder 3-ethoxy-4-methoxybenzaldehyde with a purity of 99.9% and a yield of 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com