Preparation method of negative liquid crystal compound
A technology of negative liquid crystals and compounds, which is applied in the field of preparation of negative liquid crystal compounds, can solve problems such as limited application range, poor miscibility, and poor stability, and achieve the effects of cost reduction, less impurities, and moderate refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, preparation
[0052] (1) Put 58.5g of 2,3-difluorophenetole (compound a-1), 42g of potassium tert-butoxide and 500ml of tetrahydrofuran into a 1000ml four-necked bottle. Under the protection of nitrogen, lower the temperature to -90~-100°C, add 200ml of 2.5mol / L butyllithium solution dropwise, and then keep the temperature for 1.5h. Add 30g of N,N-dimethylformamide dropwise at -90~-100°C. Slowly rise to room temperature and stir for 3h. The feed solution was poured into hydrochloric acid ice water for hydrolysis, extracted with ethyl acetate, dried, and passed through a silica gel column to obtain 4-ethoxy-2,3-difluorobenzaldehyde (compound 1-1) with a purity of 97.5% (GC), Yield 84%. Its reaction formula is as follows:
[0053]
[0054] (2) Put 170g of methyl bromide triphenylphosphine salt and 500ml of tetrahydrofuran into a 1000ml four-necked bottle. Under the protection of nitrogen, 58 g of potassium tert-butoxide was added at 0°C, and then k...
Embodiment 2
[0059] Embodiment 2, preparation
[0060] The preparation of intermediate B-1 is the same as in Example 1. Then, 18.4g of compound b-2, 19g of 4-ethoxy-2,3-difluorostyrene (intermediate B-1), and 400ml of DMSO were added into a 500ml four-necked flask. Under the protection of nitrogen, the temperature was lowered to 0°C, 1.2 g of potassium tert-butoxide was added, and the temperature was kept for 12 hours. Pour into acid water for hydrolysis, extract with ethyl acetate, neutralize, dry, and evaporate the solvent to dryness. Use petroleum ether to pass through a silica gel column, and crystallize with ethanol to obtain a negative liquid crystal compound represented by formula (I-9), with a purity of 99.5% and a yield of 50%. Its reaction formula is as follows:
[0061]
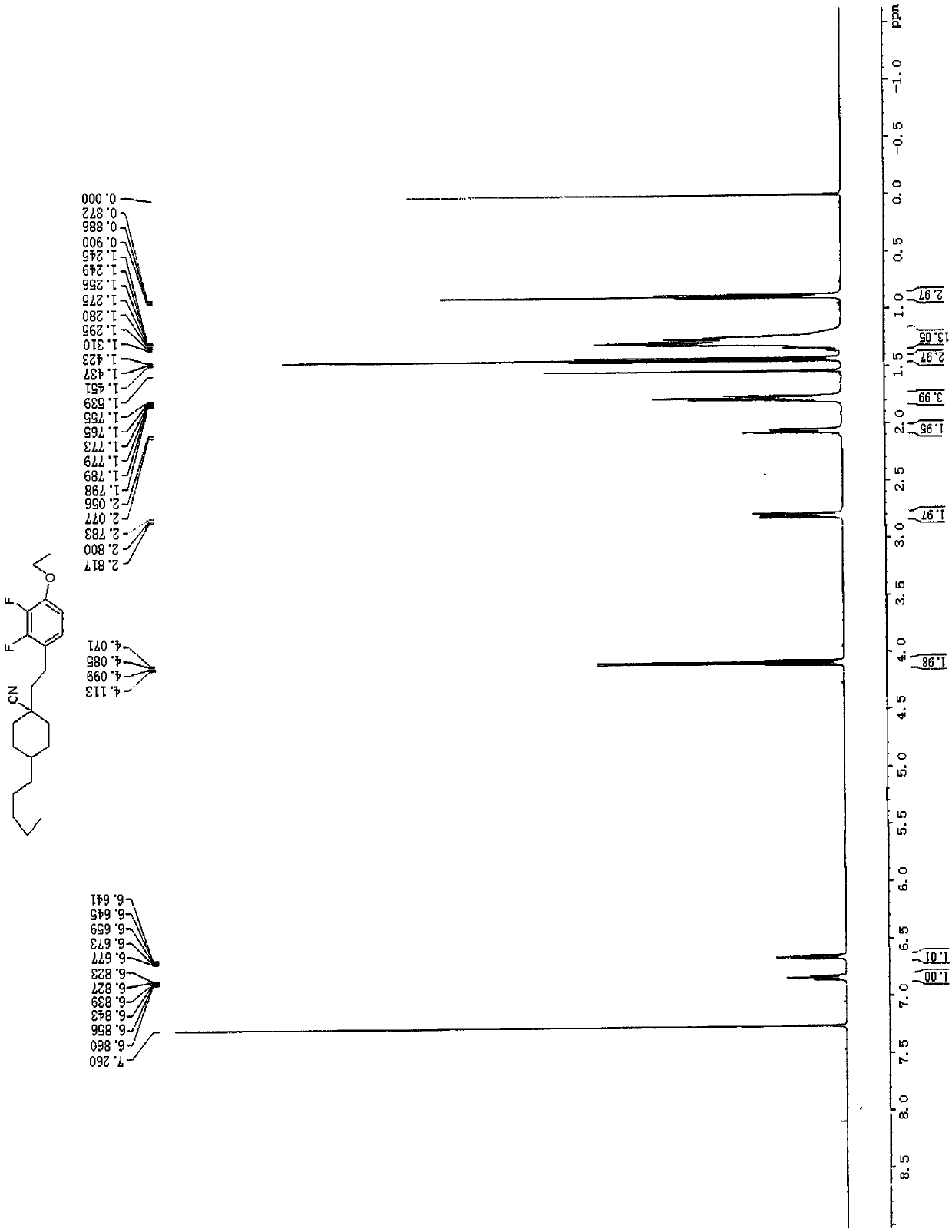
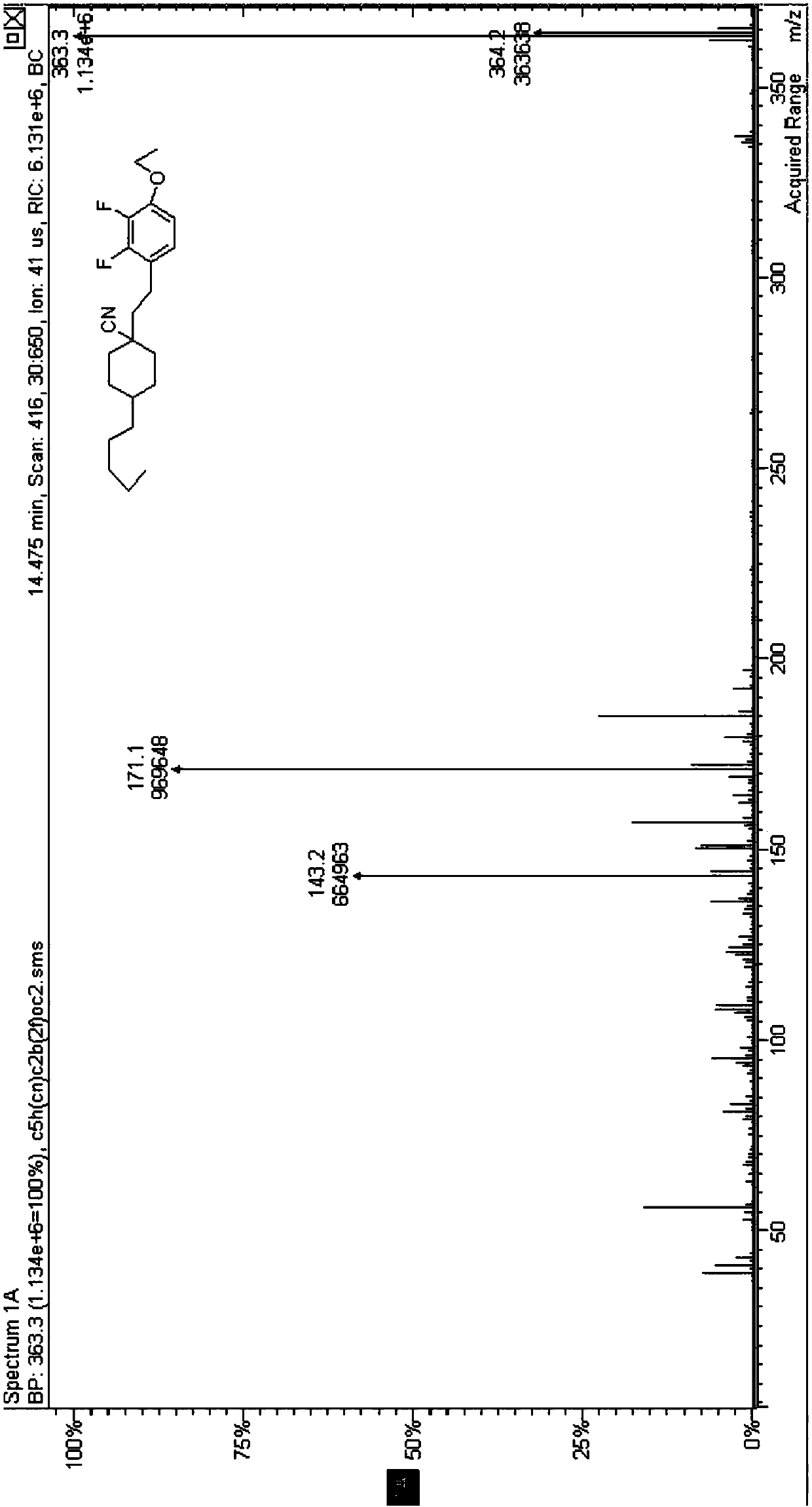
[0062] The structure of the negative liquid crystal compound shown in the formula (I-9) was confirmed, and its hydrogen nuclear magnetic resonance spectrum is as follows image 3 As shown, its mass spe...
Embodiment 3
[0063] Embodiment 3, preparation
[0064] (1) Put 58.5g of compound a-3, 42g of potassium tert-butoxide and 500ml of tetrahydrofuran into a 1000ml four-necked bottle. Under the protection of nitrogen, lower the temperature to -90~-100°C, add 200ml of 2.5mol / L butyllithium solution dropwise, and then keep the temperature for 1.5h. Add 30g of N,N-dimethylformamide dropwise at -90~-100°C. Slowly rise to room temperature and stir for 3h. The feed solution was poured into hydrochloric acid ice water for hydrolysis, extracted with ethyl acetate, dried, and passed through a silica gel column to obtain compound 1-3 with a purity of 97.5% (GC) and a yield of 84%. Its reaction formula is as follows:
[0065]
[0066] (2) Put 170g of methyl bromide triphenylphosphine salt and 500ml of tetrahydrofuran into a 1000ml four-necked bottle. Under the protection of nitrogen, 58 g of potassium tert-butoxide was added at 0°C, and then kept for 15 minutes. A solution of 80 g of compound 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com