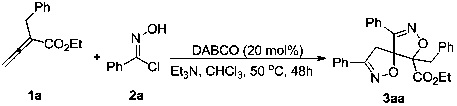Synthetic method for spiro-cycle isoxazoline compound
A technology of spirocyclic isoxazoline and synthesis method, which is applied in the synthesis field of preparing spirocyclic isoxazoline compounds, can solve the problems of heavy metal environmental pollution, application restriction and the like, and achieves stable performance, high yield and wide sources. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
[0015] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. Substrate 1a (0.05 mmol, 10.1 mg), substrate 2a (0.25 mmol, 38.8 mg), triethylamine (0.25 mmol, 25.3 mg), DABCO (0.01 mmol, 1.12 mg) were successively weighed into the reaction tube, and the Nitrogen, and chloroform (0.15 mL) was added under nitrogen atmosphere, and reacted at 50° C. for 48 hours. After the reaction was detected by TLC, silica gel was directly added, and column chromatography was spin-dried to obtain a white solid 3aa (73%). 1 H NMR (CDCl 3, 500 MHz): δ(ppm) 7.73-7.72 (m, 2H), 7.51-7.39 (m, 6H), 7.36-7.26 (m,7H), 4.29-4.16 (m, 2H), 3.99 (δ, J = 20.0 Hz, 1H), 3.65 (δ, J = 20.0 Hz, 1H),3.37 (δ, J = 15.0 Hz, 1H), 2.99 (δ, J = 15.0 Hz, 1H), 1.17 (t, J = 10.0 Hz,3H). 13 C NMR (CDCl 3 , 125 mHz): Δ (PP) 166.8, 157.1, 156.6, 133.5, 130.9,130.5, 130.5, 129.1, 129.0, 128.2, 127.5, 126.9, 93.5, 62.1, 38.3, 14.0. HRMSSS (ESI): exact mass calculateδ for M + (C 27 h ...
Embodiment 2
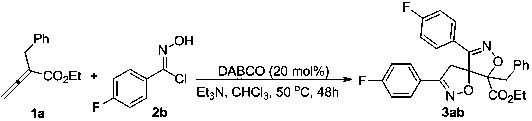
[0017]
[0018] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. Substrate 1a (0.05 mmol, 10.1 mg), substrate 2b (0.25 mmol, 38.8 mg), triethylamine (0.25 mmol, 25.3 mg), DABCO (0.01 mmol, 1.12 mg) were successively weighed into the reaction tube, and the Nitrogen, and chloroform (0.15 mL) was added under nitrogen atmosphere, and reacted at 50°C for 48 hours. After the reaction was detected by TCL, directly add silica gel, spin dry column chromatography, and obtain white solid 3ab (87%). 1 HNMR (CDCl 3, 500 MHz): δ (ppm) 7.73-7.71 (m, 2H), 7.40-7.38 (m, 2H), 7.30-7.26(m,5H), 7.17-7.14 (m, 2H), 7.05-7.02 (m, 2H), 4.27-4.18 (m, 2H), 3.98 (δ, J =15.0 Hz, 1H), 3.57 (δ, J = 15.0 Hz, 1H), 3.37 (δ, J = 15.0 Hz, 1H), 2.98 (δ, J = 15.0 Hz, 1H), 1.17 (t, J = 10.0 Hz, 3H). 13 C NMR (CDCl 3 , 125 MHz): δ (ppm)166.8, 164.3 ( J = 1005.0 Hz), 164.1 ( J = 1005.0 Hz), 156.2, 155.6, 133.3,130.5, 129.1 ( J = 30.0 Hz), 129.0 ( J = 35.0 Hz), 128.4, 1...
Embodiment 3
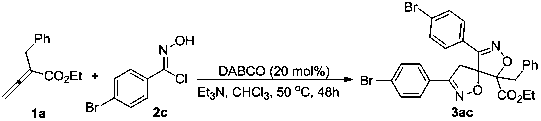
[0020]
[0021] In a 25 mL test tube reactor, exchange the air with nitrogen 3 times. Substrate 1a (0.05 mmol, 10.1 mg), substrate 2c (0.25 mmol, 38.8 mg), triethylamine (0.25 mmol, 25.3 mg), DABCO (0.01 mmol, 1.12 mg) were successively weighed into the reaction tube, and the Nitrogen, and chloroform (0.15 mL) was added under nitrogen atmosphere, and reacted at 50°C for 48 hours. After the reaction was detected by TCL, silica gel was directly added, and column chromatography was spin-dried to obtain a white solid 3ac (58%). 1 HNMR (CDCl 3, 500 MHz): δ (ppm) 7.62-7.57 (m, 4H), 7.49-7.47 (m, 2H), 7.29-7.26(m, 5H), 7.25-7.23 (m, 2H), 4.28-4.17 (m, 2H), 3.97 (δ, J = 20.0 Hz, 1H),3.54 (δ, J= 20.0 Hz, 1H), 3.36 (δ, J = 15.0 Hz, 1H), 2.98 (δ, J = 15.0 Hz,1H), 1.17 (t, J = 10.0 Hz, 3H). 13 C NMR (CDCl 3 , 125 mHz): Δ (PP) 166.7,156.2, 155.8, 133.2, 132.4, 132.3, 130.5, 128.5, 128.3, 127.6, 126.9,125.8, 125.2, 99.7, 62.7, 38.0, 14.0, 14.0, 14.0 . HRMS (ESI): exactmas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com