Technological method for preparing 2, 3, 4, 5-tetrachloride phthalic anhydride
A technology of tetrafluorobenzoic acid and tetrafluorophthalic acid, which is applied in two fields, can solve the problems of difficult process control, low product purity, and high production cost, and achieve the effects of low cost, improved reaction purity, and high competitive advantage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
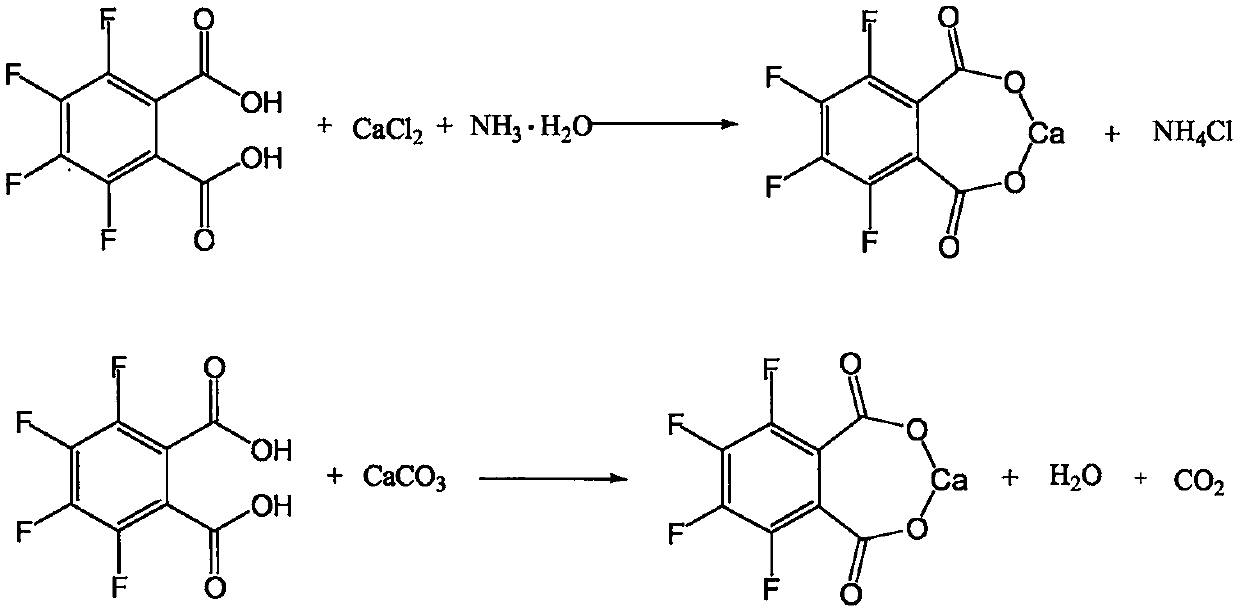
[0030] Step 1: Preparation of calcium 3,4,5,6-tetrafluorophthalate.
[0031] Add 24g of 3,4,5,6-tetrafluorophthalic acid, 200g of drinking water, and 25g of calcium chloride into a four-necked flask equipped with stirring, thermometer, and condenser, and raise the temperature to 50-55°C to dissolve all the solids , and kept stirring at this temperature for 3 hours. Then slowly add 20% ammonia solution dropwise until the pH gradually approaches neutrality. When the pH value reaches 6.0-7.0, stop the dropwise addition. After stirring at 50-55°C for 30 minutes, retest the pH and the reaction ends.
[0032] Step 2: Preparation of calcium 2,3,4,5-tetrafluorobenzoate.
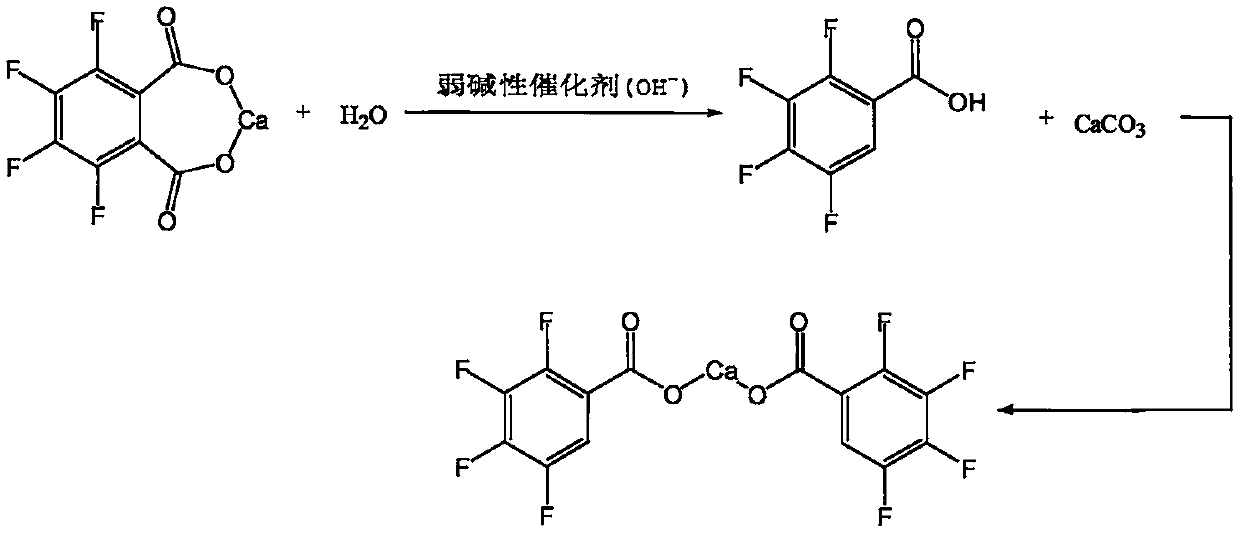
[0033] Slowly lower the temperature of the above reaction solution to 5-10°C, solids will gradually precipitate out, keep stirring at this temperature for 2 hours, filter, wash with appropriate amount of cold water, and obtain 3,4,5,6-tetrafluorophthalate calcium wet material About 32g. Throw the solid back into a...
Embodiment 2
[0040] Step 1: Preparation of calcium 3,4,5,6-tetrafluorophthalate.
[0041] Put 24g of 3,4,5,6-tetrafluorophthalic acid, 200g of drinking water, and 35g of calcium chloride into a four-neck flask in turn, raise the temperature to 60-65°C to dissolve all the solids, and keep stirring at this temperature for 2 ~3 hours. Then slowly add 20% ammonia solution dropwise, and when the pH value reaches 6.0-7.0, stop the dropwise addition, and use about 88 g of deammonia water. After stirring at 60-65°C for 30 minutes, retest the pH and it remains unchanged.
[0042] Step 2: Preparation of calcium 2,3,4,5-tetrafluorobenzoate.
[0043]Cool the reaction solution to 5-10°C, solids gradually precipitate out, and keep stirring at this temperature for 2 hours, then filter, wash with appropriate amount of cold water, and obtain 3,4,5,6-tetrafluorophthalate calcium wet material of about 33.5 g. Put the solid wet material back into a four-necked flask, add about 250g of drinking water and 3...
Embodiment 3
[0050] Step 1: Preparation of calcium 3,4,5,6-tetrafluorophthalate.
[0051] Put 48g of 3,4,5,6-tetrafluorophthalic acid, 400g of drinking water, and 80g of calcium bromide into a four-neck flask successively, raise the temperature to 60-65°C to dissolve all the solids, and keep stirring at this temperature for 2 ~3 hours. Then slowly add 20% aqueous ammonia solution dropwise, and when the pH value reaches 6.0-7.0, stop the dropwise addition, and use about 175 g of 20% concentration ammonia water. After stirring at 60-65° C. for 30 minutes, retest the pH unchanged.
[0052] Step 2: Preparation of calcium 2,3,4,5-tetrafluorobenzoate.
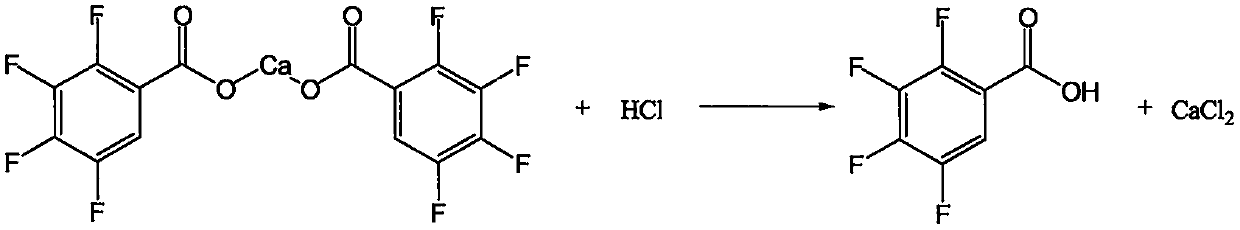
[0053] Cool the reaction solution to 5-10°C, solids gradually precipitate out, and keep stirring at this temperature for 2 hours, then filter, wash with appropriate amount of cold water, and obtain 3,4,5,6-tetrafluorophthalate calcium wet material of about 65.8 g. Put the solid wet material back into a four-necked flask, add about 1000g of dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com