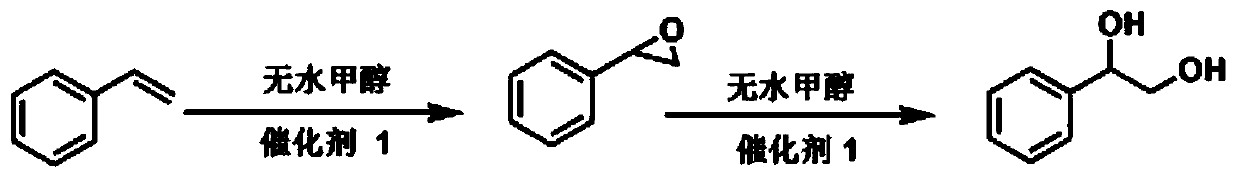
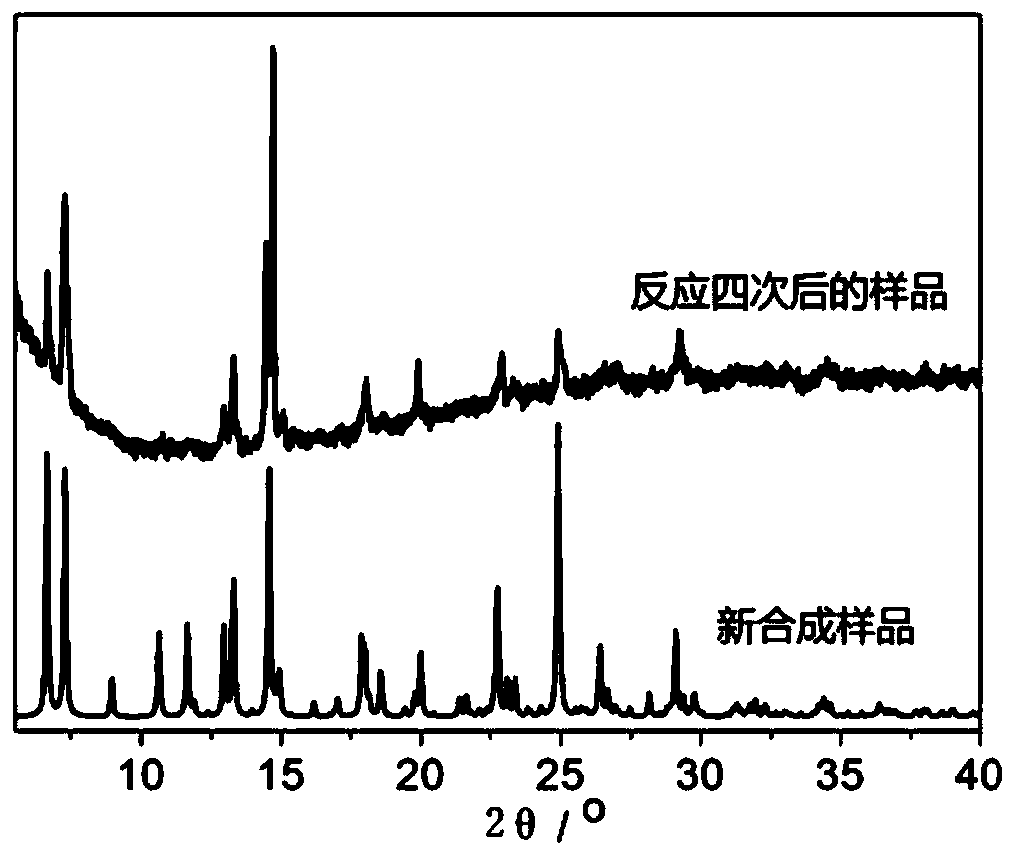
A catalyst for one-pot preparation of 1-phenyl-1,2-ethylene glycol from styrene, preparation method and application thereof
A catalyst, styrene technology, applied in organic chemistry methods, compounds containing elements of Group 8/9/10/18 of the periodic table, chemical instruments and methods, etc. The effect of large conjugated system, good catalytic activity and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Synthesis of Co(II) crystalline heterogeneous catalysts
[0030] (1) Weighing according to the molar ratio of 2,4,6-tris(4-pyridyl)-1,3,5-triazine (tpt) to 5-aminoisophthalic acid is 1:1, and after mixing, it becomes a mixture Ligand;
[0031] (2) According to Co(NO 3 ) 2 .6H 2 Weigh Co(NO 3 ) 2 .6H 2 O;
[0032] (3) Mix and dissolve the reagents weighed in (1) and (2) in 8 ml of isopropanol / water (2:2 by volume) solvent under stirring to prepare a precursor solution;
[0033] (4) Transfer the precursor solution obtained in (3) into a hydrothermal kettle, perform solvothermal reaction at 100° C. for 48 hours, suction filter, wash, and dry to obtain a three-dimensional structure Co(II) crystalline heterogeneous catalyst.
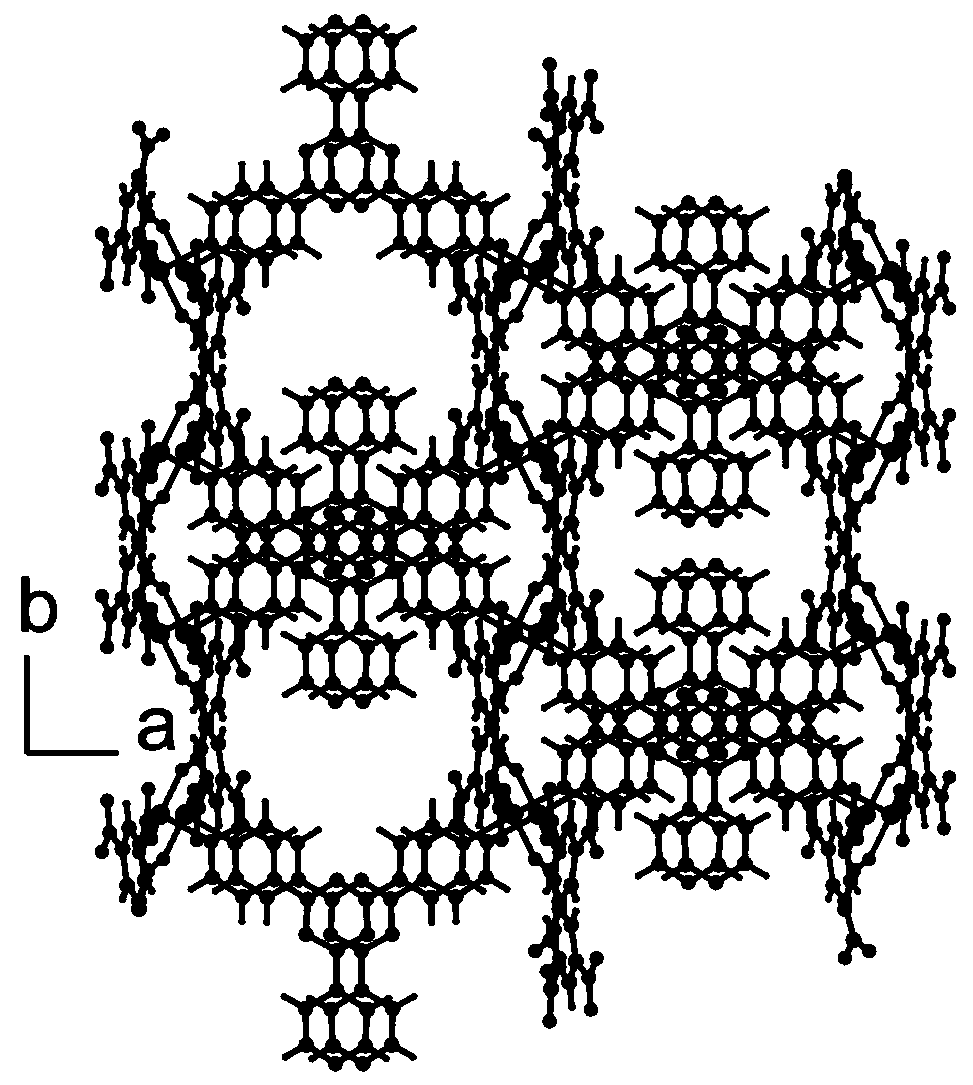
[0034] Use the Bruker APEX II diffractometer to collect single crystal diffraction intensity data to obtain the microscopic coordination structure of the catalyst material, such as figure 1 It is a three-dimensional structure diagram of the...
Embodiment 2
[0039] 1. Synthesis of Co(II) crystalline heterogeneous catalysts
[0040] (1) Weighing according to the molar ratio of 2,4,6-tris(4-pyridyl)-1,3,5-triazine (tpt) to 5-aminoisophthalic acid is 1:1, and after mixing, it becomes a mixture Ligand;
[0041] (2) According to Co(NO 3 ) 2 .6H 2 Weigh Co(NO 3 ) 2 .6H 2 O;
[0042] (3) Mix and dissolve the reagents weighed in (1) and (2) in 8 ml of isopropanol / water (2:2 by volume) solvent under stirring to prepare a precursor solution;
[0043] (4) Transfer the precursor solution obtained in (3) into a hydrothermal kettle, perform solvothermal reaction at 120° C. for 72 hours, suction filter, wash, and dry to obtain a three-dimensional structure Co(II) crystalline heterogeneous catalyst.
[0044] The single crystal diffraction intensity data was collected on the Bruker APEX II diffractometer to obtain the microscopic coordination structure of the catalyst material, in which the metal-centered Co(II) ion was bridged by two carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com