Adrenocorticotropic hormone solution and application thereof
A technology of adrenal cortex and hormones, applied in the direction of material inspection products, coatings, instruments, etc., can solve problems such as high requirements for instruments and equipment, increase product production costs, and affect test results, etc., to reduce preparation costs and facilitate operation and preparation 、Overcome the complex effect of freeze-drying process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 adrenocorticotropic hormone solution
[0022] The corticotropic hormone solution of the present invention comprises the following steps:
[0023] (1) Dissolve 2-morpholineethanesulfonic acid in water, then add NaCl, after complete dissolution, add NaOH or hydrochloric acid to adjust the pH value to 5.4-5.6;
[0024] (2) Add trehalose, casein, 4-aminoantipyrine, surfactant, glycerol, and biological preservatives to the solution in step (1) in sequence, and then add NaOH or hydrochloric acid to adjust the pH value to 5.5-6.7 ; pH preferably 5.9-6.1.
[0025] (3) Adding corticotropin antigen to the solution in step (2).
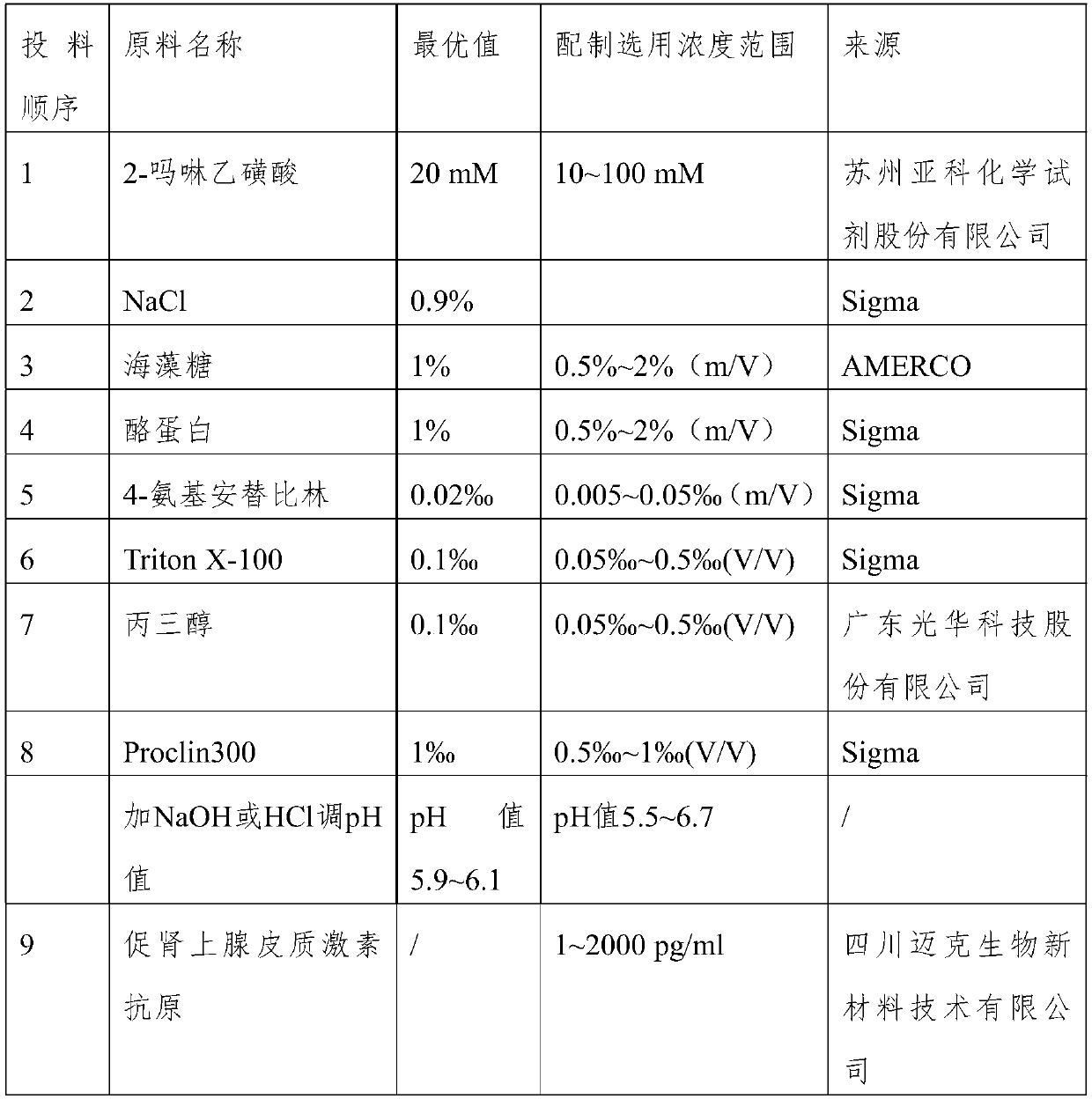
[0026] The dosage and source of specific ingredients are shown in Table 1. Purified water is used as the dosing solvent, and the other material can only be put in after the former material is completely dissolved.
[0027] Table 1
[0028]
[0029]
Embodiment 2
[0030] Performance evaluation of embodiment 2 corticotropic hormone solution
[0031] Stability tests were carried out using five different formulations of groups A, B, C, D, and E in Table 2. Wherein group B is the optimal ratio of each component of the corticotropic hormone solution of the present invention, group C is the higher concentration ratio of each component of the corticotropic hormone solution of the present invention, and group D is the corticotropic hormone solution of the present invention The concentration ratio of each component is lower, and the ratio and pH value of the other components in group E are the same as those in group B except that 4-aminoantipyrine is not added.
[0032] Table 2
[0033]
A
B
C
D
E
Phosphate buffer
20mM
/
/
/
/
2-Morpholineethanesulfonic acid
/
20mM
100mM
10mM
20mM
NaCl
0.90%
0.90%
0.90%
0.90%
0.90%
1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com