Preparation of a dual-excitation fluorescent probe and its application in the detection of hydrazine in water
A fluorescent probe, fluorescein technology, applied in the field of analytical chemistry, can solve problems such as liver central nervous system damage, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
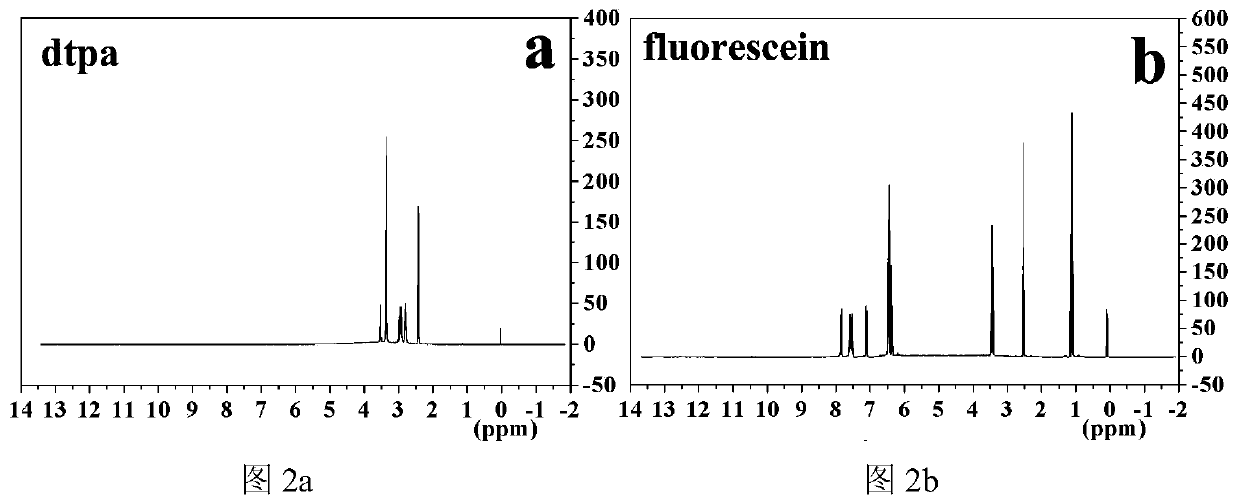
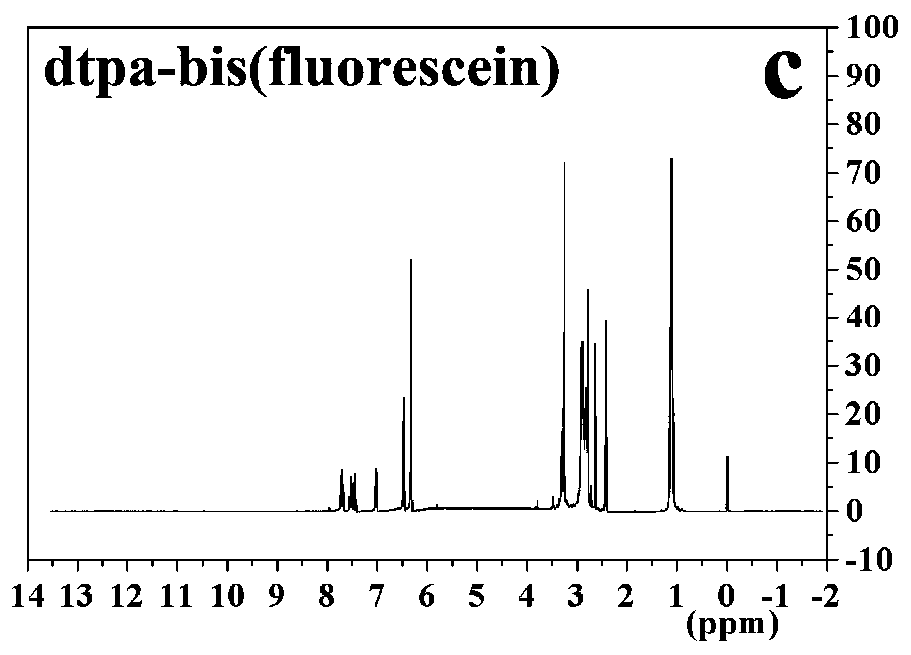
[0034] Embodiment 1 Novel dual excitation fluorescent probe Tb III -dtpa-bis(fluorescein)
[0035] (1) Preparation method
[0036] 1, the preparation of diethylenetriaminepentaacetic dianhydride (dtpaa)
[0037] Weigh 7.8100g (0.02mmol) of dtpa, 16.0mL (0.08mmol) of acetic anhydride, and 10.0mL (0.12mmol) of pyridine into a three-necked round bottom flask, mix well, and heat with stirring at 65°C for 24h. Cool to room temperature, filter the reaction mixture, wash twice with a small amount of acetic anhydride and anhydrous ether, filter with a vacuum pump, and dry the resultant in a vacuum oven at 80°C to obtain dtpaa.
[0038] 2. Preparation of diethylenetriaminepentaacetic acid difluorescein (dtpa-bis (fluorescein))
[0039] Take 1.9610g of dtpaa (5.5mmol), 8.0mL (16.5mmol) of triethylamine, anhydrous DMF (50mL), and 1.6515g (11mmol) of fluorescein in a three-neck round bottom flask, and mix well. Heating at a constant temperature of 100°C and stirring rapidly for 24 hou...
Embodiment 2
[0047] Embodiment 2 Double excitation fluorescent probe Tb 3+ -Application of dtpa-bis(fluorescein) in detection of hydrazine
[0048] 1. Fluorescence spectra of dual-excitation fluorescent probe hydrazine detection
[0049] Experimental conditions: Take a certain amount of hydrazine (N 2 h 4 ) was prepared with distilled water to a concentration of 1.0×10 -3 mol / L solution as a hydrazine stock solution.
[0050] Take 5.0 mL of the hydrazine stock solution in a 50 mL volumetric flask, and then add 5.0 mL of the dual-excitation fluorescent probe stock solution prepared in Example 1, and use buffer to make up the volume. At this time, the probe concentration was 5.0×10 -5 mol / L, the concentration of each detected substance is 1.0×10 -4 mol / L. Changes in fluorescence spectra were observed at excitation wavelengths of 247 nm and 500 nm.
[0051] The result is as Figure 5a , Figure 5b shown. like Figure 5a As shown, under the excitation of 247nm, the fluorescent prob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com