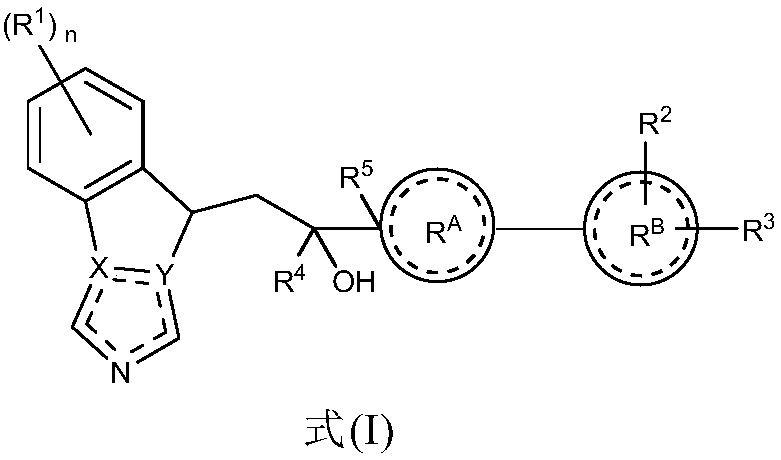
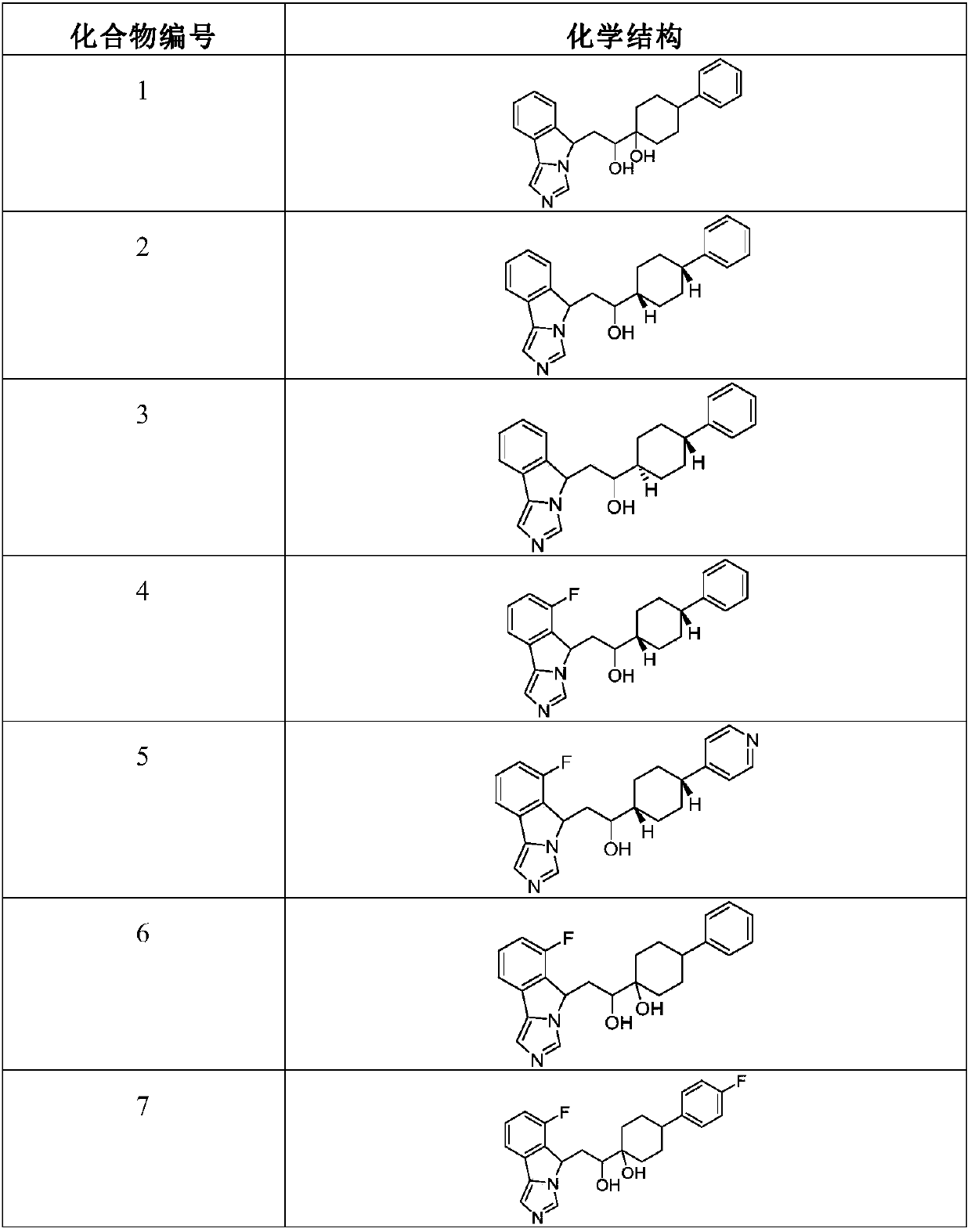
Indoleamine 2,3-dioxygenase inhibitor and uses thereof in pharmacy
A pharmaceutical, amine-based technology, applied in the field of chemical compounds, can solve problems such as weak level activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0136] The preparation of prodrugs is well known in the art and described, for example, in King, F.D., ed., Medicinal Chemistry: Principles 15 and Practice, The Royal Society of Chemistry, Cambridge, UK (2nd edition, reproduced, 2006); Testa, B. et al., Hydrolysis in Drug and Prodrug Metabolism. Chemistry, Biochemistry and Enzymology, VCHA and Wiley-VCH, Zurich, Switzerland (2003); Wermuth, C.G., ed., The Practice, of Medicinal Chemistry, 3rd edition, Academic Press, San Diego, CA (2008).
[0137] The present invention is intended to include all isotopes of atoms present in the compounds of the present invention. Isotopes include atoms with the same atomic number but different mass numbers. By way of general example and not limitation, isotopes of hydrogen include deuterium and tritium. Isotopes of carbon include 13C and 14C. Isotopically-labeled compounds of the present invention may be prepared generally by conventional techniques known to those skilled in the art or by m...
Embodiment 1
[0217] Example 1 Preparation of 2-(1-trityl-1H-imidazol-4-yl)benzaldehyde
[0218]
[0219] 2-formylphenylboronic acid (1.55g, 6.88mmol), 1-trityl-4-iodoimidazole (3.0g, 4.59mmol), potassium phosphate (4.38g, 13.77mmol), Pd(PPh 3 ) 4 (0.3g, 10%, w / w%) was added to a mixed solution of DMF (30mL) and water (6mL), the system was replaced with nitrogen 4 times, and reacted overnight at 90°C under nitrogen protection. TLC detected that the reaction was complete, cooled to room temperature, filtered, the filtrate was diluted with ethyl acetate (300mL), washed with water (100mL) three times, the organic phase was evaporated under reduced pressure to remove the solvent, and the residue was separated by flash column chromatography to obtain 2.15g of the target product , the yield was 75.6%. 1 H NMR (CDCl 3 ,400MHz)δ10.44(s,1H),7.94 (dd,J 1 =0.8Hz,J 2 =0.8Hz,1H),7.65-7.63(m,1H),7.58-7.53(m,2H),7.40-7.36(m,10H),7.18-7.21(m,6H),7.04(d,J=1.2 Hz, 1H).
Embodiment 2
[0220] Example 2 Preparation of 1-(1-hydroxyl-4-phenylcyclohexyl)ethyl-1-one
[0221]
[0222] 4-Phenylcyclohexanone (0.26g, 1.5mmol) was dissolved in tetrahydrofuran (1.0mL), nitrogen was replaced three times, diiodine solution (0.1M in THF, 15mL, 1.5mmoL) was slowly added dropwise, and then Acetyl chloride (0.39g, 1.0mmoL) was slowly added dropwise to the reaction flask. After the drop was completed, the reaction was stirred overnight at room temperature. After the reaction was complete by TLC, it was quenched with 0.1M hydrochloric acid. Volatile substances were evaporated under reduced pressure, and the residue was separated by flash column chromatography to obtain 0.16 g of the target product with a yield of 52%. 1 H NMR (DMSO-d 6 , 400MHz) δ7.31-7.15 (m, 5H), 5.20 (s, 1H), 2.20 (s, 3H), 1.82-1.77 (m, 2H), 1.65-1.59 (m, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com