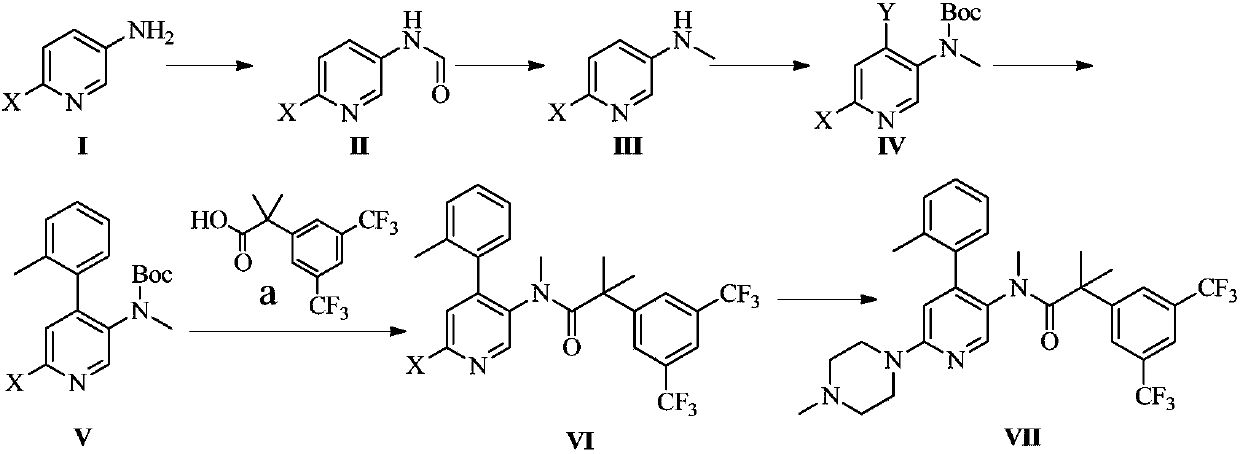
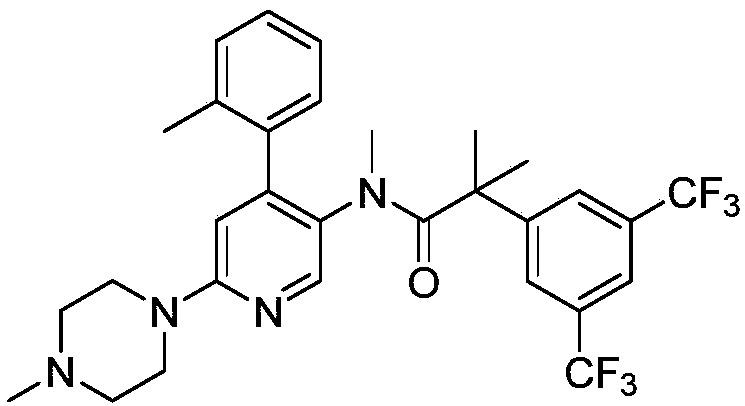
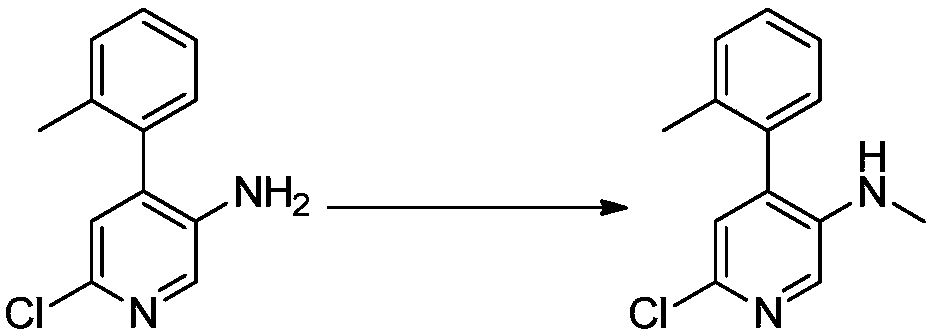
Method for preparing netupitant
A netupitant and compound technology, applied in the field of preparation of netupitant, can solve the problems of high toxicity and complex operation, and achieve the effects of less environmental pollution, simple post-treatment, and improved reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 The preparation method of netupitant of the present invention
[0048] 1. Preparation of Compound II
[0049]Add 2-chloro-5-aminopyridine (compound Ⅰ) (45.0g, 350mmol) to formic acid (75mL, 1980mmol), stir and react at reflux for 2h, evaporate the formic acid under reduced pressure, and add 300ml of saturated sodium bicarbonate solution to the residue , and then extracted twice each time with ethyl acetate 600ml, combined the organic layers, dried with anhydrous sodium sulfate, filtered, and spin-dried to obtain oily compound II (52.1g, 95%).
[0050] 2. Preparation of Compound III
[0051] Compound II (52.0g, 332mmol) was dissolved in THF (300ml), lithium tetrahydrogen aluminum (25.2g, 664mmol) was slowly added in batches at room temperature, stirred for 6h, and sodium sulfate decahydrate was added to the reaction solution until the precipitation was complete After filtration, the filtrate was concentrated to dryness to obtain oily compound III (41.0 g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com