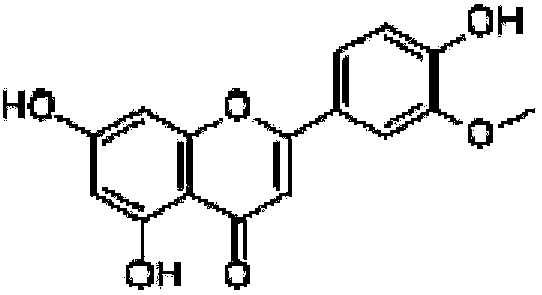
Compounding method for chrysoeriol
The technology of a golden eriodroxanthin and a synthetic method is applied in the field of synthesis of golden eriodroxanthin, which can solve the problems of large reaction odor, low cyclization yield, complex post-treatment, etc., and achieves simple steps, simple post-treatment, The effect of easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
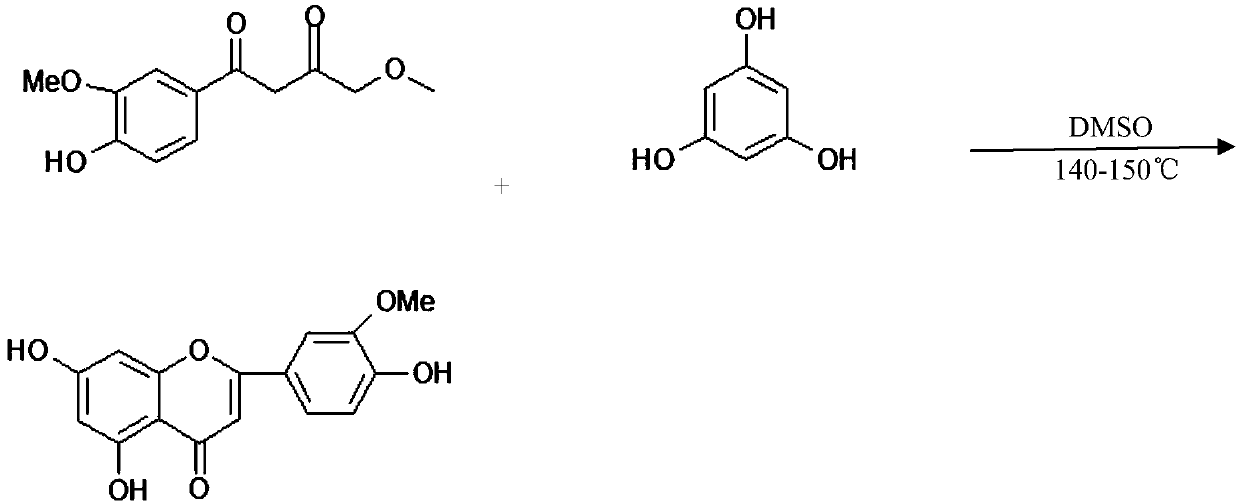
[0029] Put 12g of anhydrous phloroglucinol, 24g of ethyl 3-methoxy-4-hydroxy-benzoylacetate, and 120g of DMSO in a 250ml beaker, heat up and stir in a vacuum for 12 hours, then stop the heating, remove the vacuum and cool to room temperature Then add 200ml of water, stir for 12 hours, and filter to obtain 32g of solid crude eriodine.
[0030] Recrystallize the crude product with 15 times the volume of new ethanol, add 10% of the mass of the crude product to decolorize with activated carbon, remove the activated carbon, concentrate the ethanol to one-fifth of the original volume, place it for crystallization, filter and dry to obtain 98% golden eriodictine 29 grams.
Embodiment 2
[0032] Put 6g of anhydrous phloroglucinol, 12g of 3-methoxy-4-hydroxy-ethyl benzoylacetate, and 60g of DMSO in a 250ml beaker, heat up and stir in a vacuum reaction for 12 hours, then stop heating, remove the vacuum and cool to room temperature Then add 100ml of water, stir for 12 hours, and filter to obtain 15g of solid crude eriodine.
[0033] Recrystallize the crude product with 15 times the volume of new ethanol, add 10% of the mass of the crude product to decolorize with activated carbon, remove the activated carbon, concentrate the ethanol to one-fifth of the original volume, place it for crystallization, filter and dry to obtain 98% golden eriodictine 13 grams.
Embodiment 3
[0035] Put 24g of anhydrous phloroglucinol, 48g of 3-methoxy-4-hydroxy-ethyl benzoylacetate, and 240g of DMSO in a 500ml beaker, heat up and stir in a vacuum for 12 hours, then stop the heating, remove the vacuum and cool to room temperature Then add 400ml of water, stir for 12 hours, and filter to obtain 65g of solid crude eriodine.
[0036] Recrystallize the crude product with 15 times the volume of new ethanol, add 10% of the mass of the crude product to decolorize with activated carbon, remove the activated carbon, concentrate the ethanol to one-fifth of the original volume, place it for crystallization, filter and dry to obtain 98% golden eriodictine 63 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com