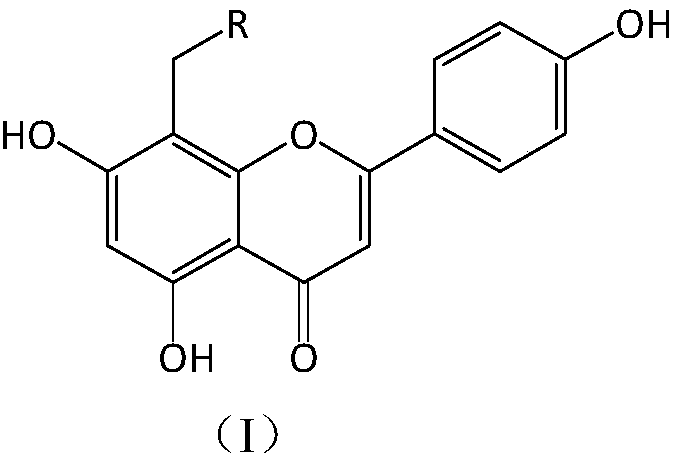
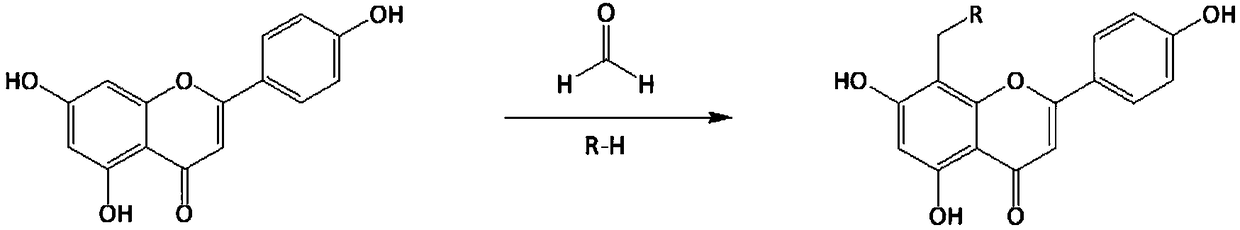
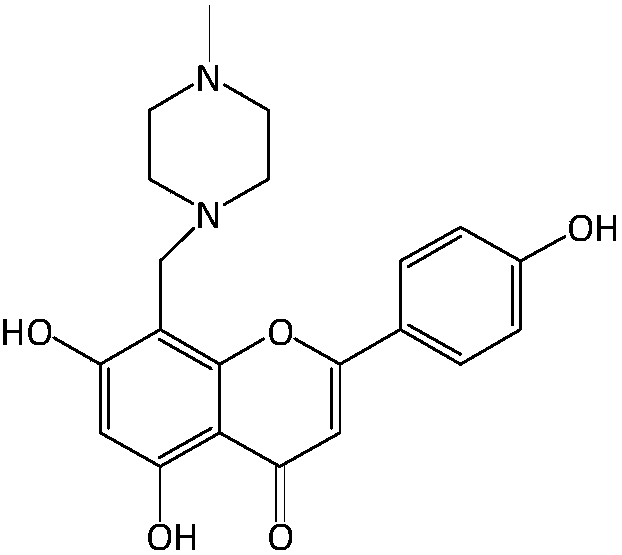
Apigenin derivatives and their application in the treatment of hyperuricemia
A technology of hyperuricemia and apigenin, applied in drug combination, bone diseases, organic chemistry, etc., can solve problems such as many side effects, and achieve the effects of improved bioavailability, increased water solubility, and good oil-water partition coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 compound I-1
[0026] Take 2.70g apigenin (10mmol) in the reaction flask, add 50ml of dimethylformamide to dissolve it, add 1.50ml (15mmol) of N-methylpiperazine, then add 1.23ml (15mmol) of 37% aqueous formaldehyde, and mix Evenly, react for 60 minutes at a temperature of 45°C in a microwave synthesizer with a set power of 350 watts to obtain a reaction solution; cool the reaction solution to room temperature, add 50ml of water and 100ml of ethyl acetate for extraction, ethyl acetate solution, no Dehydrated with sodium sulfate, the ethyl acetate solution was evaporated to dryness under reduced pressure, the residue was dissolved in acetone and allowed to stand at room temperature, and the precipitate was recrystallized with acetone to obtain compound I-1.
[0027] Compound I-1 8-(4-methylpiperazine-1-methyl)-5,7-dihydroxy-2-(4-phenol)-4H-1-benzopyran-4-one, light yellow Crystallization, yield: 68.81%; 1 H-NMR (CD 3 OD,300MHz):δ2.53(q,...
Embodiment 2
[0030] The preparation of embodiment 2 compound 1-2
[0031] Get 2.70g apigenin (10mmol) in the reaction flask, add dimethylformamide 50ml to make it dissolve, add N ethylpiperazine 1.71ml (15mmol), then add 37% formaldehyde aqueous solution, 1.23ml (15mmol), mix Evenly, react for 60 minutes at a temperature of 45°C in a microwave synthesizer with a set power of 350 watts to obtain a reaction solution; cool the reaction solution to room temperature, add 50ml of water and 100ml of ethyl acetate for extraction, ethyl acetate solution, no Dehydrated with sodium sulfate, the ethyl acetate solution was evaporated to dryness under reduced pressure, the residue was dissolved in acetone, left at room temperature, and the precipitate was recrystallized with acetone to obtain compound I-2.
[0032] Compound I-2 8-(4-ethylpiperazine-1-methyl)-5,7-dihydroxy-2-(4-phenol)-4H-1-benzopyran-4-one, light yellow Crystallization, yield: 67.22%; 1 H-NMR (CD 3 OD,300MHz):δ2.20(s,3H,-CH 3 ),2.36...
Embodiment 3
[0035] The preparation of embodiment 3 compound 1-3
[0036]Take 2.70g apigenin (10mmol) in the reaction flask, add 50ml of dimethylformamide to dissolve it, add 1.92ml (15mmol) of 1-isopropylpiperazine, then add 1.23ml (15mmol) of 37% formaldehyde solution , mixed evenly, and reacted for 60 minutes at a temperature of 45°C in a microwave synthesizer with a set power of 350 watts to obtain a reaction solution; the reaction solution was cooled to room temperature, extracted with 50ml of water and 100ml of ethyl acetate, and the ethyl acetate solution , dehydrated with anhydrous sodium sulfate, the ethyl acetate solution was evaporated to dryness under reduced pressure, the residue was dissolved in acetone, left at room temperature, and the precipitate was recrystallized with acetone to obtain compound I-3.
[0037] Compound I-3 8-(4-isopropylpiperazine-1-methyl)-5,7-dihydroxy-2-(4-phenol)-4H-1-benzopyran-4-one, shallow Yellow crystal, yield: 69.78%; 1 H-NMR (CD 3 OD,300MHz):...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com