Application of microencapsulated astaxanthin to preparation of product for preventing and treating gout
A technology of astaxanthin and microencapsulation, which is applied in microcapsules, nanocapsules, capsule delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of astaxanthin 1% microcapsule powder
[0031] Add water to 75kg of modified starch to form a solution with a solid content of 40%, add 3kg of sodium ascorbate and stir to dissolve. Take 11.4kg of astaxanthin oleoresin with a content of 10.5%, add 1.5kg of mixed tocopherols, and stir evenly. Add the astaxanthin oleoresin into the water phase of the wall material under the stirring condition, stir evenly, homogenize twice under high pressure under 50 MPa pressure, and spray dry to obtain astaxanthin 1% CWS powder.
Embodiment 2
[0032] Example 2 Preparation of astaxanthin 2% microcapsule powder
[0033] Add 66kg of modified starch and water to form a solution with a solid content of 40%, add 4kg of sodium ascorbate, and stir to dissolve 1.5kg of ascorbic acid. Take 22.5kg of astaxanthin oleoresin with a content of 10.6%, add astaxanthin oleoresin into the water phase of the wall material under stirring conditions and stir evenly, homogenize under high pressure for 2 times under 55MPa pressure, and spray dry to obtain astaxanthin 2% CWS powder .
Embodiment 3
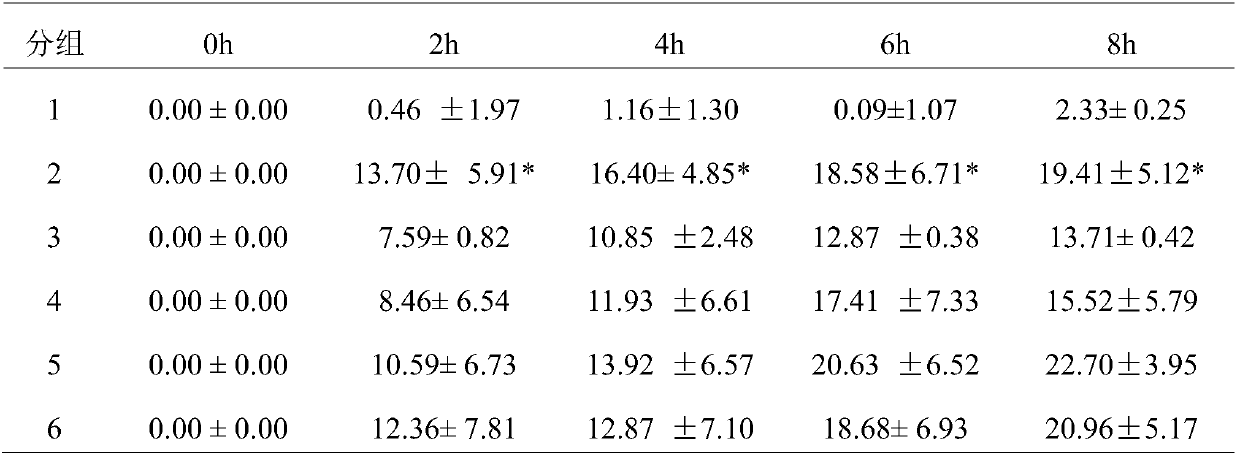
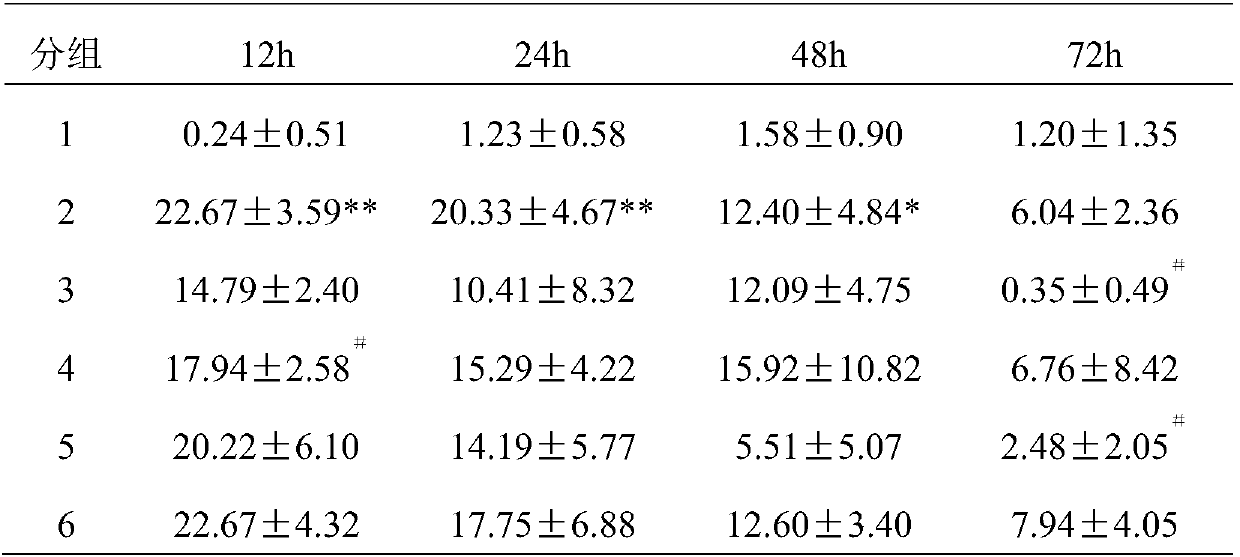
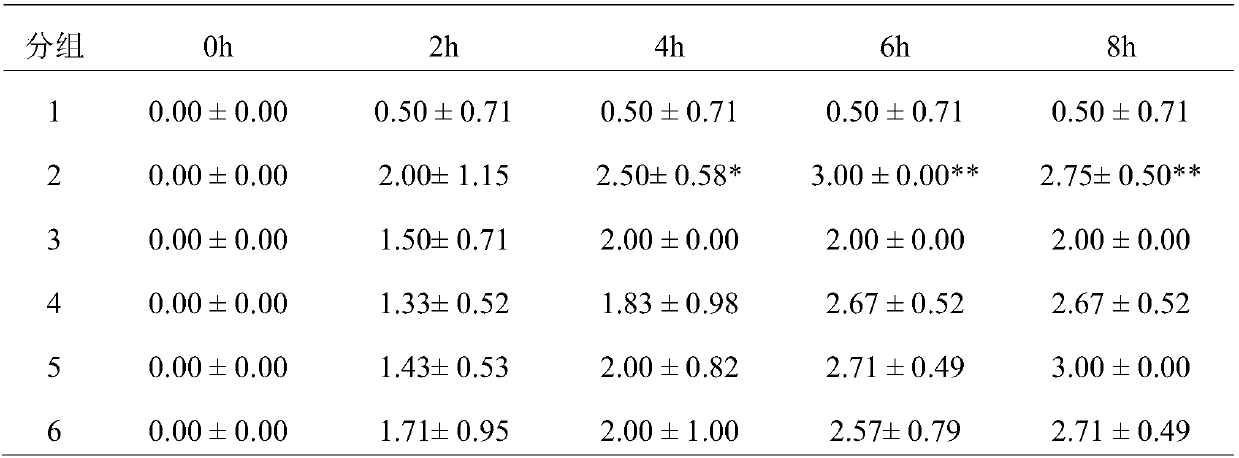
[0035] 48 rats of the present invention were adapted to 7 days in the animal room environment before the test, and were randomly divided into 6 groups, 8 of the blank control group (1 group), 8 of the GA model group (2 groups), and 8 of the colchicine group (3 groups). group) 8 rats, high-dose group (group 4) 8 rats, middle-dose group (group 5) 8 rats, low-dose group (group 6) 8 rats. Rats in each group were administered by intragastric administration for 8 days continuously, and the administration volume was as follows: 1ml / 100g, and the model was established 1h after the last administration. The rats in the colchicine group were given 1.5 mg / kg colchicine solution by intragastric administration, and the targeted drug 1 groups were given 144 mg / kg, 72 mg / kg, and 36 mg / kg by intragastric administration, respectively. Solution, continued to administer for 3 days after modeling. Rats were injected with 50 μl of 5% sodium urate solution into the tibiotarsal joint cavity of the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com