Kit for measuring and diagnosing free and cell binding total IgE in human whole blood and preparation method
A kit and free-type technology, which is applied in the field of diagnostic kits and preparations for the determination of free-type and cell-bound total IgE in whole blood, can solve the problems of low detection sensitivity, avoid inaccurate data, and achieve good clinical promotion and detection convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A diagnostic kit for measuring free and cell-bound total IgE in whole blood, comprising:
[0039] 96-well plate coated with IgE antibody.
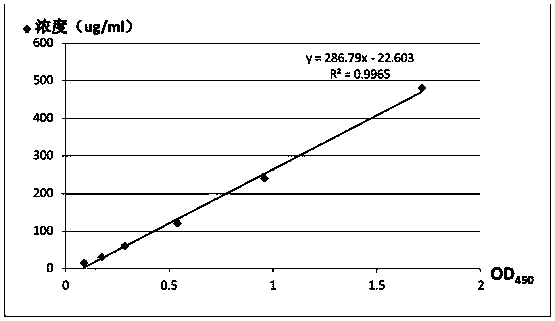
[0040] Standard. The concentration of the standard is as follows: 480, 240, 120, 60, 30, 15 μg / mL.
[0041] Sample diluent.
[0042] Detection Antibody-HRP.
[0043] 20x wash buffer.
[0044] Chromogenic substrate A.
[0045] Chromogenic Substrate B.
[0046] stop solution.
[0047] Sealing film.
[0048] user's Guide.
[0049] Configure the above kits by the following steps:
[0050] 96-well plate coated with IgE antibody. Each well was coated with 100 ng of anti-human IgE antibody.
[0051] Standard. The concentration of the standard is as follows: 480, 240, 120, 60, 30, 15 μg / mL.
[0052] Sample diluent. 1L sample diluent contains: potassium dihydrogen phosphate (KH2PO4): 0.27g, disodium hydrogen phosphate (Na2HPO4): 1.42g, sodium chloride (NaCl): 8g, potassium chloride (KCl) 0.2g, 4g BSA, pH7.4.
[0053] Detection ...
Embodiment 2
[0056] A method for using the kit prepared in Example 1, comprising the steps of:
[0057] Take out the required strips from the aluminum foil bag after equilibrating at room temperature for 20 minutes, and seal the remaining strips with a ziplock bag and put them back at 4°C.
[0058] Set standard wells and sample wells, and add 50 μL of standard substances of different concentrations to each standard well;
[0059] Add 50 μL of the sample to be tested into the sample well; do not add to the blank well.
[0060] Except for the blank wells, add 100 μL of horseradish peroxidase (HRP)-labeled detection antibody to each well of standard wells and sample wells, seal the reaction wells with plate sealing film, and incubate for 60 min in a 37°C water bath or incubator.
[0061] Discard the liquid, pat dry on absorbent paper, fill each well with washing solution (350 μL), let it stand for 1 min, shake off the washing solution, pat dry on absorbent paper, and repeat the plate washing...
Embodiment 3
[0065] Performance testing of the kit prepared by the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| sedimentation rate | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com