Method for synthesizing 4-hydroxymethyl-biphenyl
A technology for hydroxymethyl biphenyl and biphenyl, which is applied in the field of synthesizing 4-hydroxymethyl biphenyl, can solve problems such as low cost, and achieve the effects of strong price advantage, cheap and easily available raw materials, and easy control of conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
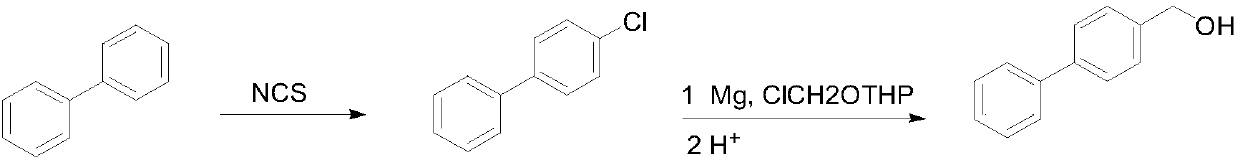
[0022] Preparation of 4-chlorobiphenyl
[0023] In a 500 mL round bottom flask, 30.8 g of biphenyl was dissolved in 250 mL of tetrahydrofuran, and 28.1 g of chlorosuccinimide was added at room temperature, and then reacted at room temperature for 36 hours. After the reaction was completed, the solvent was concentrated under reduced pressure (recovered for the next reaction), the residue was poured into water, extracted with dichloromethane, washed with water, evaporated to remove the solvent, and then recrystallized with 95% ethanol to obtain a white solid 28.3 g, yield 75%.
[0024] Preparation of 4-hydroxymethylbiphenyl
[0025] Add 4.0g metal magnesium chips, a small amount of iodine and 40mL tetrahydrofuran (recovered in the previous step) into a 500mL three-necked flask, heat to 60°C, and add dropwise 28.3g of 4-chlorobiphenyl dissolved in 130mL tetrahydrofuran (recovered in the previous step) and 13.9g of n-butyl chloride solution, dropwise, reflux reaction...
Embodiment 2
[0027]
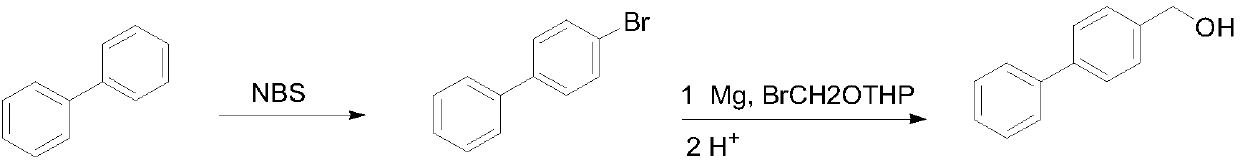
[0028] Preparation of 4-bromobiphenyl
[0029] In a 500 mL round bottom flask, 30.8 g of biphenyl was dissolved in 250 mL of 2-methyltetrahydrofuran, 35.6 g of bromosuccinimide was added at room temperature, and then reacted at room temperature for 36 hours. After the reaction was completed, the solvent was concentrated under reduced pressure (recovered for the next reaction), the residue was poured into water, extracted with dichloromethane, then washed with water, the solvent was evaporated, and then recrystallized with 95% ethanol to obtain a white solid 35.4 g, yield 76%.
[0030] Preparation of 4-hydroxymethylbiphenyl
[0031] Add 4.0g metal magnesium chips, a small amount of iodine and 40mL 2-methyltetrahydrofuran (recovered in the previous step) into a 500mL three-necked flask, heat to 60°C, and add dropwise the solution dissolved in 180mL 2-methyltetrahydrofuran (recovered in the previous step) under nitrogen protection. 35.4g of 4-bromobiphenyl and 1.4g ...
Embodiment 3
[0033]
[0034] Preparation of 4-chlorobiphenyl
[0035] In a 250 mL round bottom flask, 15.4 g of biphenyl was dissolved in 125 mL of 2-methyltetrahydrofuran, 13.4 g of chlorosuccinimide was added at room temperature, and then reacted at room temperature for 36 hours. After the reaction was completed, the solvent was concentrated under reduced pressure (recovered for the next reaction), the residue was poured into water, extracted with dichloromethane, washed with water, evaporated to remove the solvent, and then recrystallized with 95% ethanol to obtain a white solid 13.8 g, yield 73%.
[0036] Preparation of 4-hydroxymethylbiphenyl
[0037] Add 1.9g metal magnesium chips, a small amount of iodine and 20mL 2-methyltetrahydrofuran (recovered in the previous step) into a 250mL three-necked flask, heat to 60°C, and dropwise add 2-methyltetrahydrofuran dissolved in 60mL (recovered in the previous step) under nitrogen protection 13.8g of 4-chlorobiphenyl and 6.8g of n-butyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com