Method for synthesizing novel fluoride-free organic phosphine ligand modified by carbonate ester
A synthesis method and carbonate technology are applied in the field of synthesis of novel carbonate-modified fluorine-free organic phosphine ligands, which can solve environmental problems, expensive fluorocarbons and other problems, and achieve the effects of improving solubility and realizing recovery and reuse.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
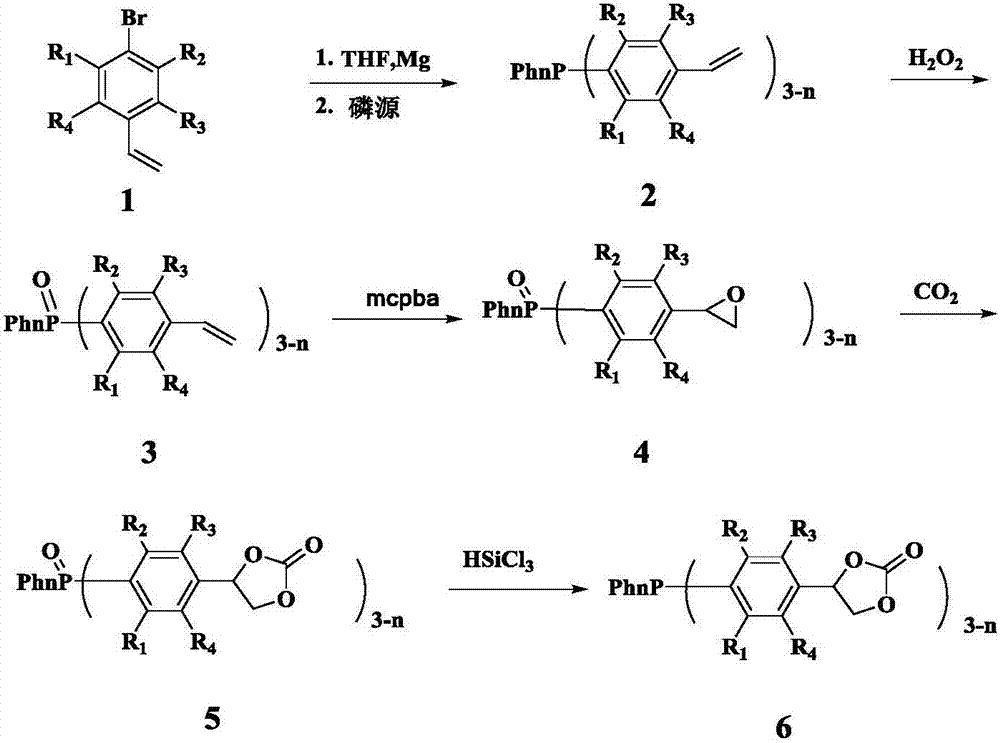
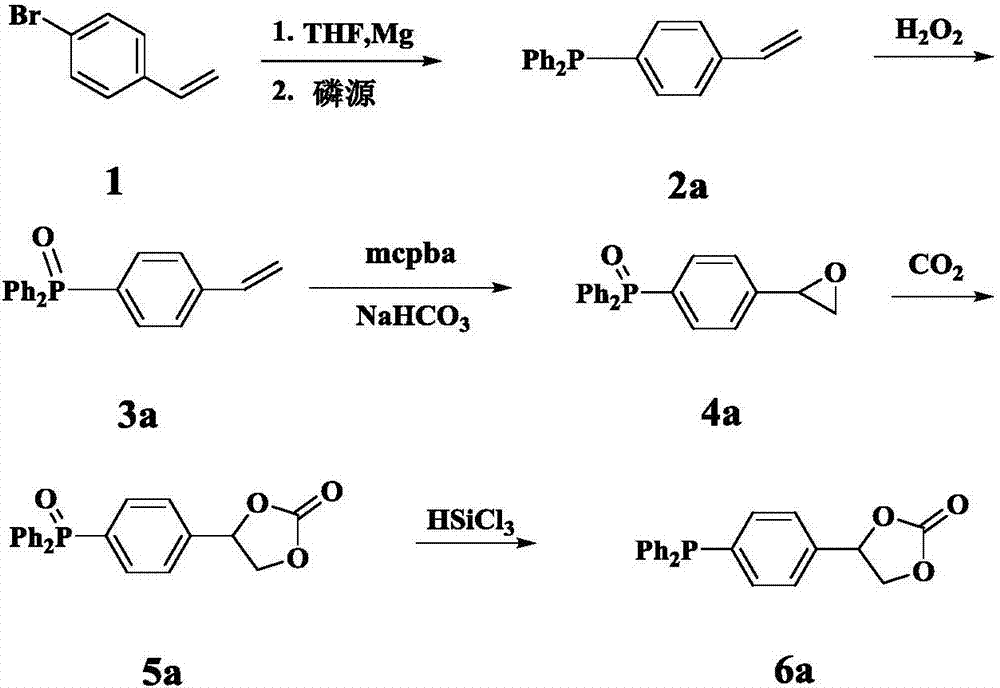
[0028] Example 1 Synthesis of (4-carbonate phenyl) diphenylphosphine compound
[0029] The first step, the synthesis of compound 2a ((4-vinylphenyl) diphenylphosphine)
[0030] Wash the magnesium powder with hydrochloric acid and acetone respectively, and drain it for later use. THF was refluxed with metal Na until the benzophenone turned dark purple, and then evaporated for later use. Add the newly prepared 1.5g Mg powder (1.1eq) into the sealed reaction device with the function of dripping reflux, and use N 2 Replace 3 times. in N 2 Under protection, sequentially add a small amount of I 2 Granules, 5mg of HQ (hydroquinone) and refined THF (about 10ml), the dried compound 1 (4-bromostyrene) (10g) was transferred to the dropping funnel, diluted with refined THF (10ml) . The reaction is initiated by heating, and after initiation, the THF solution of 4-bromostyrene is slowly added dropwise, and the rate of addition is controlled so that the temperature of the solution does...
Embodiment 2
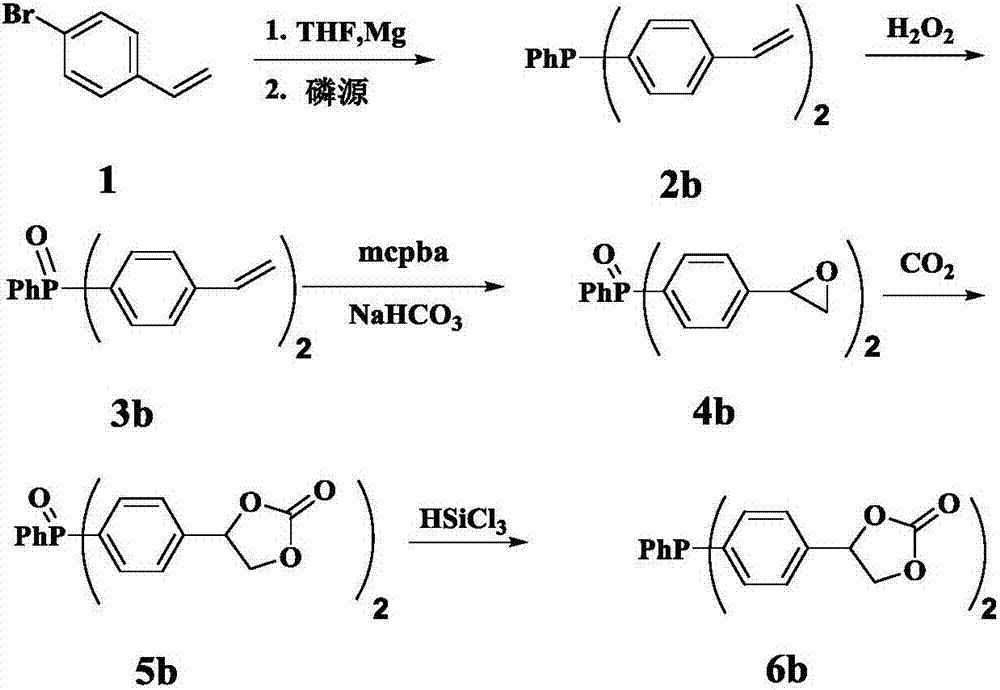
[0041] Example 2 Synthesis of (4-carbonate phenyl) diphenylphosphine compound
[0042] The first step, the synthesis of compound 2a ((4-vinylphenyl) diphenylphosphine)
[0043] Add the newly prepared Mg powder (2.0g) into the reaction device, and use N 2 Replace 3 times. in N 2 Under protection, add I in turn 2 Granules, 10 mg of HQ and 15 ml of refined THF, the dried compound 1 (4-bromostyrene) (10 g) was transferred to a dropping funnel, and diluted with refined THF. The reaction was initiated by heating, and after the initiation, a THF solution of 4-bromostyrene (10 g) was slowly added dropwise, and the rate of addition was controlled so that the temperature of the solution did not exceed 30° C. After the dropwise addition was completed, the mixture was stirred at room temperature for 2 h to obtain a gray mixture. After the Grignard reagent was prepared, the reaction apparatus was transferred to an ice-salt bath (-30° C.) and cooled and stirred for 15 minutes, and the ...
Embodiment 3
[0052] Example 3 Synthesis of (4-carbonate phenyl) diphenylphosphine compound
[0053] The first step, the synthesis of compound 2a ((4-vinylphenyl) diphenylphosphine)
[0054] The newly made Mg powder (1.8g) is added to the reaction device, and the 2 Replace 3 times. in N 2 Under protection, add I in turn 2 Granules, 15mg of HQ and 15ml of refined THF, the dried compound 1 (4-bromostyrene) (10g) was transferred to the dropping funnel, and diluted with refined THF. The reaction is initiated by heating, and after the initiation, THF solution of 4-bromostyrene (10 g) is slowly added dropwise, and the rate of addition is controlled so that the solution temperature does not exceed 30°C. After the dropwise addition was completed, the mixture was stirred at room temperature for 2 h to obtain a gray mixture. After the Grignard reagent was prepared, the reaction apparatus was transferred to an ice-salt bath (-30° C.) and cooled and stirred for 15 minutes, and the THF solution of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com