Novel gossypol Schiff base derivative with antineoplastic activity and synthetic method thereof
A synthesis method and product technology, applied in the field of medicinal chemistry, can solve the problems of drug resistance, obvious bone marrow suppression, strong toxic and side effects, etc., and achieve the effects of high utilization rate, few reaction steps, and high operational safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
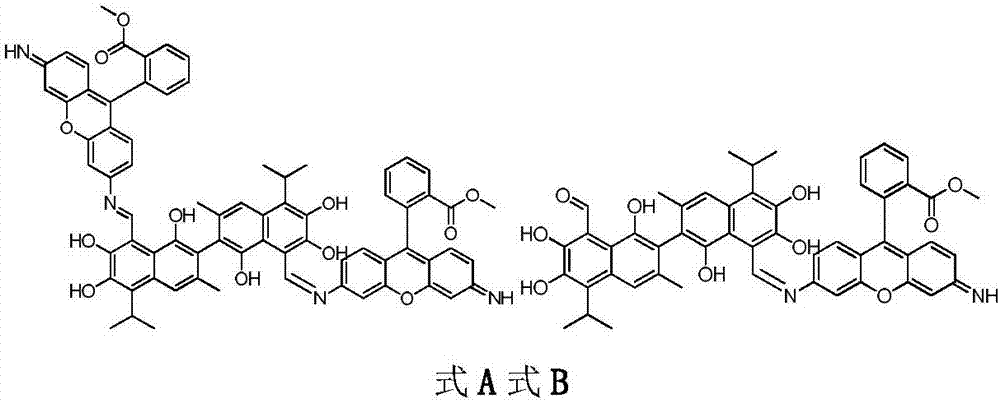
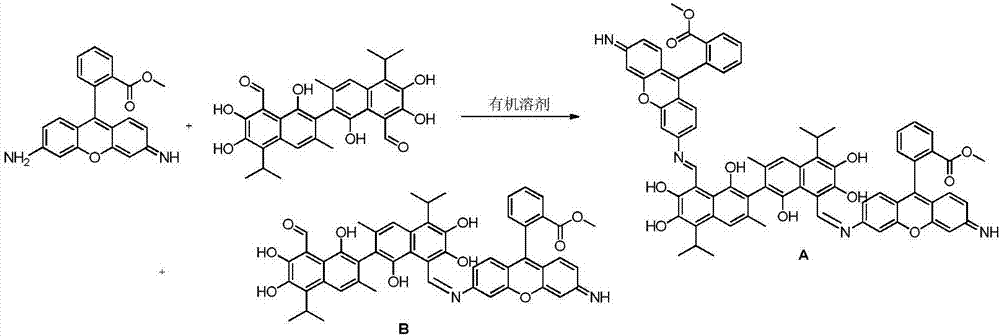
[0025] Compound A dimethyl2,2'-(6,6'-((1Z,1'E)-((1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3, 3'-dimet hyl-[2,2'-binaphthalene]-8,8'-diyl)bis(methylidene))bis(azanylylidene))bis(3-imino-3H-xanthene-9,6-diyl)) dibenzoate and compound B(Z)-methyl2-(6-(((8'-formyl-1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'- Preparation of dimethyl-[2,2'-binaphthalen]-8-yl)methylene)amino)-3-imino-3H-xanthen-9-yl)be nzoate.
[0026]
[0027] Accurately weigh 103.71mg (0.2mmol) of gossypol acetate and 131.7mg (0.4mmol) of rhodamine 123 and dissolve them in 20mL of chloroform respectively, then mix them and place them in a round-bottomed flask, raise the temperature to 40°C under magnetic stirring, and react for 12 hours. Thin-layer chromatography tracked the reaction until it was complete, stopped heating, removed the condensing device, filtered the reaction solution after cooling to obtain the crude product of mixture A and B, added 25ml of acetone to the obtained crude product for rec...
Embodiment 2
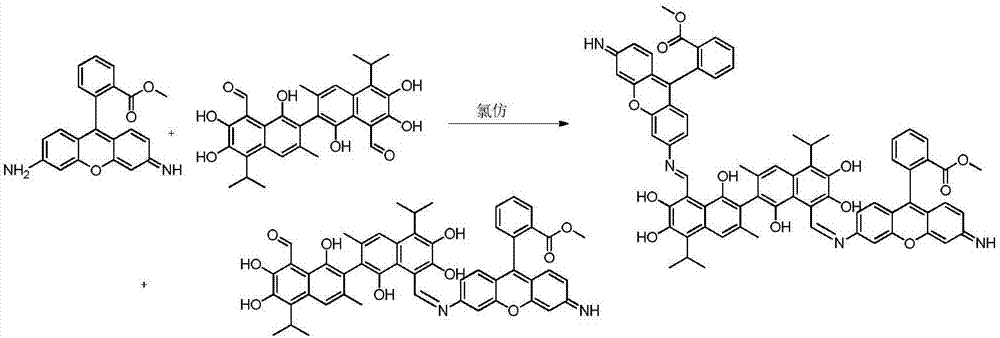
[0035]Compound A dimethyl2,2'-(6,6'-((1Z,1'E)-((1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3, 3'-dimet hyl-[2,2'-binaphthalene]-8,8'-diyl)bis(methylidene))bis(azanylylidene))bis(3-imino-3H-xanthene-9,6-diyl)) dibenzoate and compound B(Z)-methyl2-(6-(((8'-formyl-1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'- Preparation of dimethyl-[2,2'-binaphthalen]-8-yl)methylene)amino)-3-imino-3H-xanthen-9-yl)be nzoate.
[0036]
[0037] Accurately weigh 103.71mg (0.2mmol) of gossypol acetate and 158.1mg (0.48mmol) of rhodamine 123 and dissolve them in 25mL of toluene respectively, then mix them and place them in a round bottom flask, raise the temperature to 60°C under magnetic stirring, and react for 12 hours. Thin-layer chromatography tracked the reaction until it was complete, stopped heating, removed the condensing device, filtered the reaction solution after cooling to obtain the crude product of mixture A and B, added 25ml of ethanol to the obtained crude product for recrysta...
Embodiment 3
[0039] Compound A dimethyl2,2'-(6,6'-((1Z,1'E)-((1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3, 3'-dimet hyl-[2,2'-binaphthalene]-8,8'-diyl)bis(methylidene))bis(azanylylidene))bis(3-imino-3H-xanthene-9,6-diyl)) dibenzoate and compound B(Z)-methyl2-(6-(((8'-formyl-1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'- Preparation of dimethyl-[2,2'-binaphthalen]-8-yl)methylene)amino)-3-imino-3H-xanthen-9-yl)be nzoate.
[0040]
[0041] Accurately weigh 103.71mg (0.2mmol) of gossypol acetate and 168.1mg (0.5mmol) of rhodamine 123 and dissolve them in 25mL of ethyl acetate respectively, then mix them and place them in a round bottom flask, raise the temperature to 60°C under magnetic stirring, and react for 12 hours . Thin-layer chromatography tracked the reaction until it was complete, stopped heating, removed the condensing device, filtered the reaction solution after cooling to obtain the crude product of mixture A and B, added 25ml of acetone to the obtained crude product for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com