Use of Babaodan in the preparation of medicines for preventing non-alcoholic fatty liver
A non-alcoholic, fatty liver technology, applied in the field of Babao Dan in the preparation of drugs for the prevention of non-alcoholic fatty liver disease, can solve the problems of liver toxicity, lack of standardized and completely effective treatment methods, etc., and achieve definite curative effect , good development prospects, and remarkable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
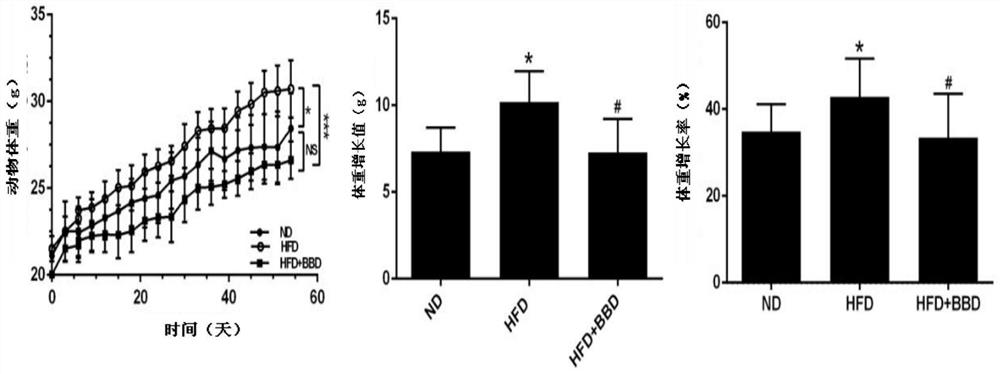
[0017] Embodiment 1 body weight and liver index
[0018] Weigh and record the mice every week, observe the body weight of the mice in each group, and compare the changes in body weight before and after the drug administration. ), body weight growth rate (%)=(final weight-initial weight) / initial weight×100%.
[0019] Determination of LEE'S index value: Accurately measure body length (length from nose to anus) and tail length under anesthesia, and then calculate LEE'S INDEX value according to the following formula:
[0020] LEE'S INDEX=weight (g)^(1 / 3)*1000 / body length (cm)
[0021] After the above experiment was over, the liver was quickly taken out and weighed to calculate the liver index (liver weight / body weight*100%).
[0022] See the experimental results figure 1 And Table 1, BBD can significantly inhibit body weight gain and reduce liver index in C57 mice.
[0023] Table 1 shows the data of body weight, Lee's index and liver index in the process of non-alcoholic lipog...
Embodiment 2
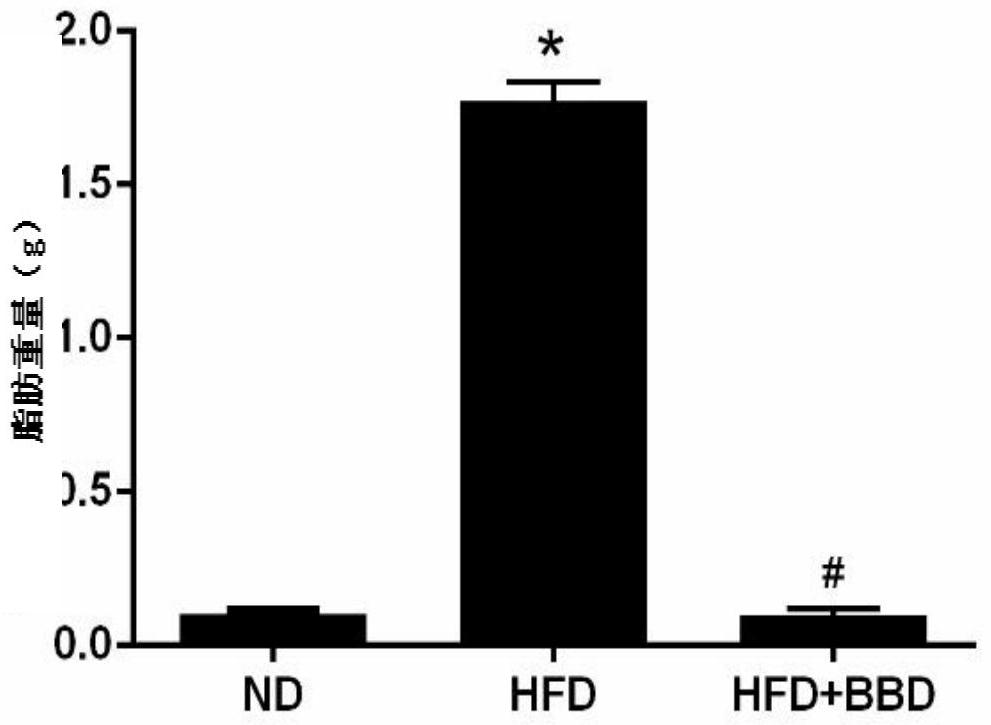
[0026] Embodiment 2 fat content
[0027] After the experiment of the growth index in Example 1 was completed, the fat accumulation around the liver was dissected to observe the fat accumulation, and the weight of the fat was weighed.
[0028] See the experimental results figure 2 , Compared with the normal group, the body fat content of the mice in the HFD group was significantly increased, and the fat content in the BBD group was significantly reduced after intragastric administration.
Embodiment 3
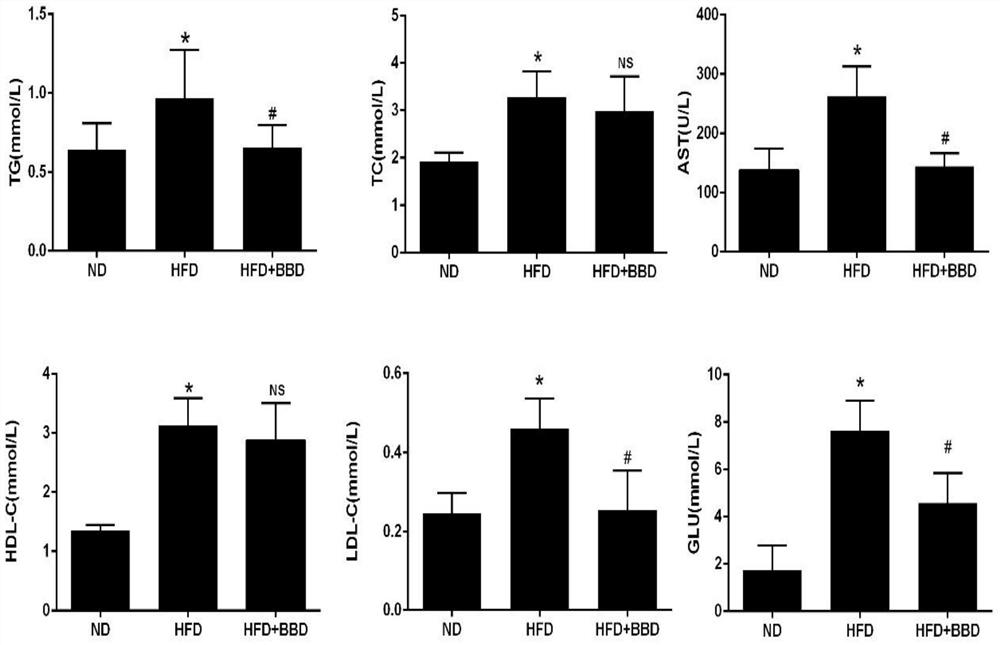
[0029] Embodiment 3 serological examination
[0030] After the experiment of the growth index in Example 1 was finished, fasted for 6 hours, blood was taken from the mice, centrifuged at 3000r / min for 10min, the separated serum was to be tested, and serum cholesterol (TC), triglyceride (TG), high-density lipoprotein were measured (HDL), low-density lipoprotein (LDL), aspartate aminotransferase (AST), blood glucose (Glu) levels.
[0031] See the experimental results image 3 , the content of each index in the serum of mice in the HFD group was significantly increased, and the TG content in the BBD group was significantly decreased after intragastric administration, and the TC content was slightly decreased. Babaodan can also reduce the elevated LDL, Glu and AST caused by fatty liver, but has no significant effect on reducing the elevated HDL.
[0032] AST is aspartate aminotransferase, which mainly exists in the mitochondria of liver cells. When the liver is severely necrotic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com