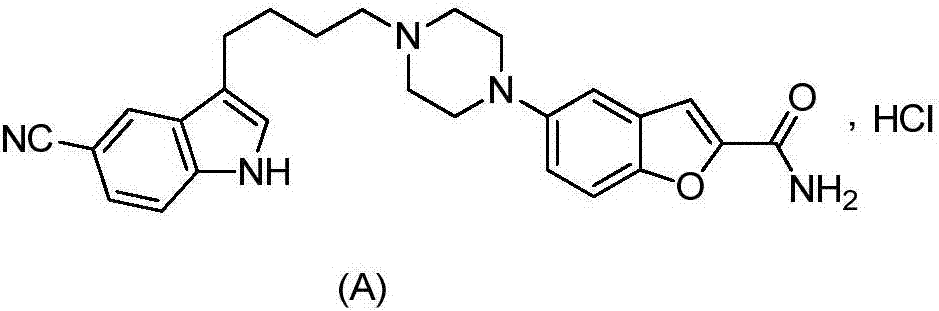
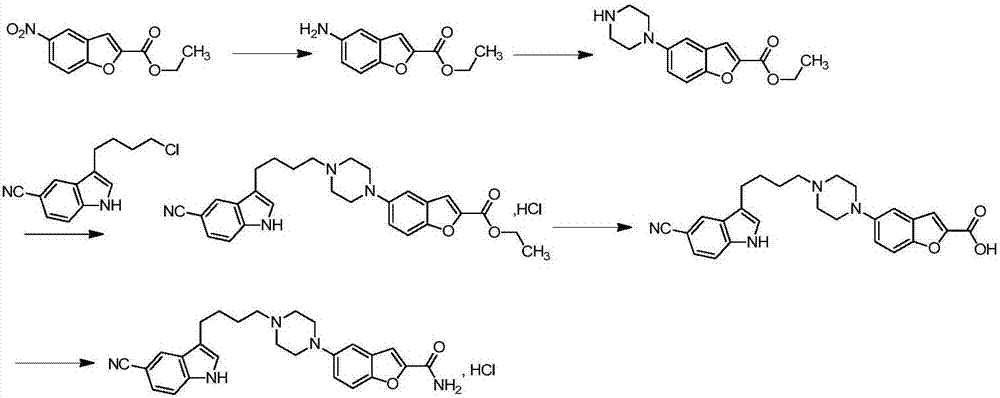
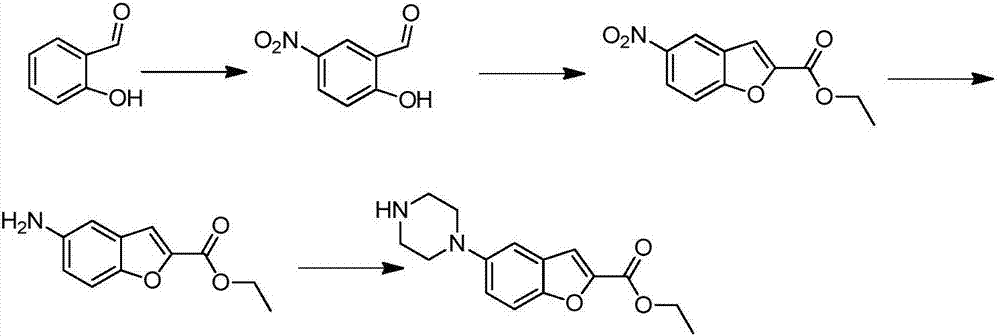
Vilazodone intermediate synthesis method
A synthesis method and vilazodone technology are applied in the field of synthesizing vilazodone intermediates, and can solve the problems of low yield, cumbersome post-processing, long reaction time and the like, so as to shorten the reaction time, simplify the production process, The effect of improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Add 19.1 g of 6-nitrocoumarin, 8.3 g of sodium hydroxide, 100 ml of ethanol, and 100 ml of water into the reaction flask, and heat to reflux for 4 hours. After the reaction was completed, dilute hydrochloric acid was added to adjust the pH to 3-4, filtered, and vacuum-dried to obtain 17.6 g of 3-(2-hydroxy-5-nitrophenyl)acrylic acid, with a yield of 84.1%.
Embodiment 2
[0033]
[0034] Dissolve 20.9 g of 3-(2-hydroxy-5-nitrophenyl)acrylic acid prepared in Example 1 in 100 ml of DMF, add 2.1 g of copper chloride and 5.0 g of cesium carbonate, and heat to 100°C for 5 hours to react. After completion, the reaction solution was poured into 300ml of ice water, filtered, and the filter cake was vacuum-dried and recrystallized with 250ml of ethanol / water mixed solvent to obtain 17.6g of 5-nitrobenzofuran-2-carboxylic acid, with a yield of 85.0%, a purity of 97.7%.
Embodiment 3
[0036]
[0037] Dissolve 20.9 g of 3-(2-hydroxy-5-nitrophenyl)acrylic acid prepared in Example 1 in 100 ml of DMSO, add 2.5 g of cuprous iodide and 5.0 g of cesium carbonate, and heat to 100° C. for 5 hours. After the reaction was completed, the reaction solution was poured into 300 ml of ice water, filtered, and the filter cake was vacuum-dried and recrystallized with 250 ml of ethanol / water mixed solvent to obtain 15.9 g of 5-nitrobenzofuran-2-carboxylic acid, with a yield of 76.7%. Purity 95.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com